November 15, 2009 (Vol. 29, No. 20)
Reduction of Sample Volumes Required Is One of the Key Benefits of DBS Assays
Toxicokinetic (TK) studies play an important role in the preclinical phase of the drug discovery process, determining the relationship between the systemic exposure of a compound in an animal model and its toxicity. Parameters such as bioavailability and dose proportionality of the compound are analyzed, from which the toxicity may be predicted and on which the doses to be used in clinical trials can be decided.
Plasma or serum samples have traditionally been used for generation of TK data due to the difficulties associated with handling whole blood. However, this approach requires relatively large volumes (100–500 µL) of blood in order to produce the required volume of matrix for bioanalysis, which makes it difficult to generate serial profiles in rodent test models. Composite profiles are therefore necessary, which can result in lower quality data and requires the use of a greater number of animals.
In recent years, dried blood spot (DBS) assays have received increasing attention as an alternative to venous blood sampling. Although the technique has been around for over 40 years and is widely used in the screening of newborn babies for metabolic abnormalities and clinical trials in remote areas, it is only now starting to be adopted in preclinical safety assessments.
Sample collection for a preclinical DBS assay involves spotting a small volume of blood (typically between 10-20 µL per sample) onto the DBS collection card (Whatman FTA DMPK from GE Healthcare). These cards consist of a specialized matrix that lyses cells on contact, denatures proteins, and inactivates bacteria and viruses. The resultant samples can then be air dried and stored or shipped at room temperature.
When required, a disc of typically 3 mm in diameter is punched from the card and the analytes isolated by liquid extraction. Analysis can then carried out by LC-MS/MS, which offers great sensitivity, analysis speed, and selectivity.
Advantages of DBS Assays
DBS assays offer many advantages in TK studies, from ease of sample collection, storage, and post-collection processing, to quality of data generated. While plasma samples must be stored in a freezer, DBS cards can routinely be stored at room temperature.
Stability may be analyte-specific and should be checked, but experience has generally been good. Successful analysis after 56 days on DBS at room temperature has been shown. In addition, the use of DBS presents a low biohazard risk, due to minimal handling of the whole blood and the inactivation of viral and bacterial contamination when the blood sample comes into contact with the card. This pathogen inactivation results from the chemical coatings of FTA DMPK-A and FTA DMPK-B cards and is not available with uncoated cellulose cards.
The number of animals required for rodent toxicity/TK studies can be drastically reduced by using this approach, as less blood is required per time point and a full time point profile can be collected from each animal. This reduction in animal use is both ethically and economically desirable. Serial sampling also enables better correlations of exposure with toxicity to be drawn, which leads to higher quality rodent TK data than would be possible with plasma or serum sampling. Figure 1 shows an example of a TK profile of dexamethaone in rats over a time period of 24 hours.
Researchers have also compared DBS sampling with the traditional whole blood sampling method and demonstrated that quantitative analysis of a drug extracted from DBS provides higher quality PK data while reducing the volume of blood required by an order of magnitude lower than the current practice used in the pharmaceutical industry.
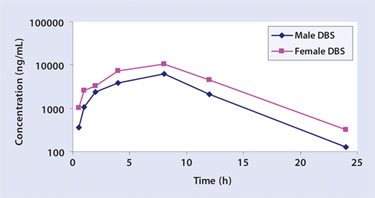
Figure 1. TK profile of dexamethasone in male and female rats over 24 hours by using DBS
Assessment of Performance
Accuracy and precision are vital elements in a TK study, and the impact of using DBS has been assessed using quality control samples at four different concentrations for both dextromethorphan (DM) and dextrophan (DX). These samples were analyzed against an LC-MS/MS calibration curve, constructed using spiked DBS standards.
The accuracy, defined as the percent deviation from the theoretical concentration, was generally within 10% for all the quality control samples. Relative standard deviation (RSD) for the replicate analyses, where n=6, was typically less than 10%. The conclusion from this data is that DBS provides the accuracy and precision required for TK studies.
In addition, two other factors that could potentially affect the quality of the resultant data were investigated—volume of blood sample applied to the card and consistency with the blood spot.
It has been found that, although a consistent blood volume can be helpful for generating more consistent results, accurate pipetting of the sample onto DBS cards is not required. When QC samples of three different concentrations of acetaminophen were taken at volumes of 10, 20, 40, and 50 µL, a small increase in accuracy was observed in only the larger 40 and 50 µL volumes (Figure 2). Therefore, it was concluded that full GLP calibration was unnecessary. Thus DBS provides a simple blood-sampling procedure that can be easily used at the site of sampling.
To assess the effect of punch position within a blood spot, QC samples punched from the center of a larger blood spot were compared to those from closer to the edge of the spot. Any variations fell within the measurement error and can be considered as having good precision with no significant difference. The results support analyte concentration homogeneity across a blood spot made with good technique on a good matrix.
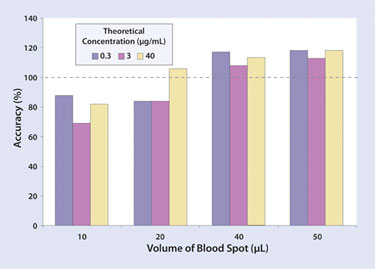
Figure 2. The accuracy of the dried blood spot method for different blood volumes for acetaminophen in dog blood
Summary
The DBS assay has been shown to be a viable method for performing TK analyses, displaying the necessary accuracy and precision for such experiments. The reduction in the required volume of blood enables collection of more samples from each test animal, thereby directly reducing the number of animals while at the same time increasing the quality of the data generated.
TK studies using DBS have the added benefit that samples can be stored and safely shipped at ambient temperatures. Using DBS not only increases the speed of testing by reducing sample processing steps, but can also contribute cost savings in preclinical TK testing.
James C. Robbins, Ph.D. ([email protected]), is field applications specialist at GE Healthcare. Lee Goodwin, Ph.D., is head of method development, and Phillip Turpin, Ph.D., is principal method development specialist at Covance.



