February 15, 2011 (Vol. 31, No. 4)
New Microscale Technology with Online Monitoring Facilitates Processes for Biofuel Applications
In recent years, many microbioreactor strategies have been developed for aerobic fermentations. Microbioreactors based on bubble columns, miniaturized stirred tanks, and simple microplates are all commonplace. These reaction platforms facilitate high-throughput operations, and in many cases, also offer online process information to advance fermentation studies in systems and synthetic biology as well as in bioprocess development.
In contrast, very few techniques have been developed for anaerobic fermentations. Available products include several microfluidic devices developed in academia and microplates, which are commonly used to study anaerobic organisms and processes.
Currently, detailed information on anaerobic fermentation processes can only be gained from fermentations in standard stirred tank reactors where an anaerobic atmosphere can easily be maintained by gassing with nitrogen or carbon dioxide. Critical process information such as online pH, temperature, and offline biomass, substrate, and product concentrations are available with these systems.
Biofuels
Biorefineries have reignited interest in anaerobic fermentations, with biobutanol production being the principle driver. Biobutanol was historically produced in Clostridium acetobutylicum, however, the production of butanol was subsequently converted to a petrochemical-based process. Due to the volatility of the oil market, the biobutanol process is having a resurgence.
This article will discuss the adaptation of m2p-labs’ BioLector® technology for anaerobic fermentations. BioLector is a high-throughput fermentation platform with online-monitoring capabilities. This technology provides 48 or 96 parallel fermentations in the standard microplate format, it also detects online biomass and fluorescent proteins as well as pH and DO values during fermentation.
As opposed to traditional stirred tank bioreactors, BioLector does not require cleaning, sterilization, or calibration procedures or tube connections. It has been demonstrated in aerobic cultures that the results of fermentations in BioLector microplates are easily scalable to stirred tank bioreactors. It can thus be inferred that the optimized process conditions in the BioLector can be rapidly transferred to larger-scale bioreactors.
In general, anaerobic fermentations can already be performed with the basic BioLector technology by connecting nitrogen or carbon dioxide to the BioLector incubation chamber. The major drawback of this simple system is that relatively large gas flows in the range of 100 mL/min are required and the applied gas can diffuse into the laboratory atmosphere.
Additionally, transfer of the microplate from the anaerobic bench to the BioLector poses the risk of gas contamination and a return to aerobic conditions. In the case of obligate anaerobic microorganisms, this situation is a knock-out criterion. To circumvent this problem, the Anaerobe Chamber for the BioLector was developed.
The Anaerobe Chamber seals the microplate completely against ambient air. A removable transparent chamber cover allows easy viewing of the microplate and the cultures and also provides access to the microplate during fermentation for sampling or feeding (Figure 1).
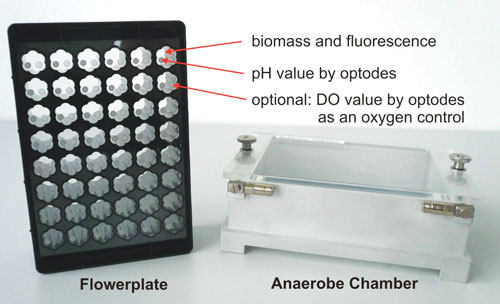
Figure 1. The Anaerobe Chamber with the multiparameter Flowerplate® for the BioLector
Use of the chamber is demonstrated in Figure 2. First, the microplate is filled with culture media and cell inoculum under an anaerobic bench. To avoid culture contamination, the microplate must be covered with a sterile, gas-permeable membrane that also allows gas exchange with the inner chamber gas atmosphere. Then, the Anaerobe Chamber is placed over the microplate to seal the microplate against ambient air.
The anaerobe atmosphere of the anaerobe bench is enclosed in the Anaerobe Chamber, which ensures the transfer of the microplate to the BioLector under anaerobic conditions. The Anaerobe Chamber is easily secured to BioLector by setting it within the centering pins, subsequently the preinstalled and nitrogen-flushed gas tubes in the BioLector are connected to the push-in connectors in the Anaerobe Chamber. After that, the system is ready to start.
Gas flow in the Anaerobe Chamber during operation is controlled by a mass flow controller and is normally set to 2 mL/min, but it can also be changed to higher gas flows if necessary. Either nitrogen or carbon dioxide can be connected to the BioLector to provide the anaerobic conditions, other applicable gas mixtures include a microaerophilic gas or a Syngas atmosphere.

Figure 2. Application principle of the Anaerobe Chamber for fermentation studies
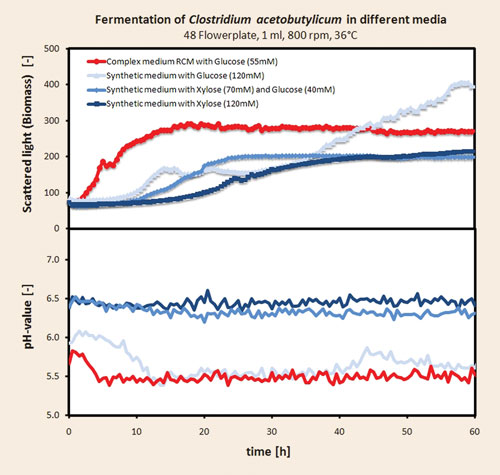
Anaerobic fermentations of Clostridium acetobutylicum (ATCC 824) can be facilitated with the Anaerobe Chamber. A study with four different media (complex and synthetic origin) with several replicates was performed in the anaerobic high-throughput fermentation system to demonstrate the utility of the Anaerobe Chamber. The results are presented in Figure 3.
A clear difference in the growth behavior in the four different applied media can be seen. Growth on the complex medium results in the fastest growth. A typical diauxic growth phase is depicted, which demonstrates the growth on glucose and on yeast extract and peptones in the later phase. The pH value on the complex medium drops rapidly from 5.8 to 5.5 during glucose consumption. The cultures on the synthetic media grew more slowly than in the complex medium. Within the synthetic media the fastest growth can be observed under pure glucose.
The cultures on xylose-containing media show decreased growth as xylose concentration was increased. The culture on pure glucose shows a prominent diauxic growth with a long second growth phase up to 60 hours and with the highest biomass concentration obtained in this experiment.
The second growth phase is more often than not carried out on organic acids that were produced on the initial glucose substrate. This assumption is supported by a strong decrease in the pH value of the pure glucose medium. The two cultures on xylose-containing media also demonstrated diauxic growth but with only a short second growth phase. Surprisingly, the pH values of the xylose-containing media decreased only slightly, thus, the acidification in this media is not prominent. This could explain the catabolite repression of Clostridium acetobutylicum on xylose-containing media that has been reported in the literature.
This small experiment confirmed the utility of using BioLector for anaerobic fermentations. This new technology can dramatically reduce complexity and effort in fermentation studies on anaerobic microorganisms by replacing large numbers of experiments in stirred tank bioreactors and shake flasks. In addition to making it possible to study a large number of clones or media in a high-throughput manner, the system also simultaneously provides detailed kinetic data on biomass growth and pH value by noninvasive online measurements, the combination of these capabilities is currently not commonplace in traditional bioreactors.
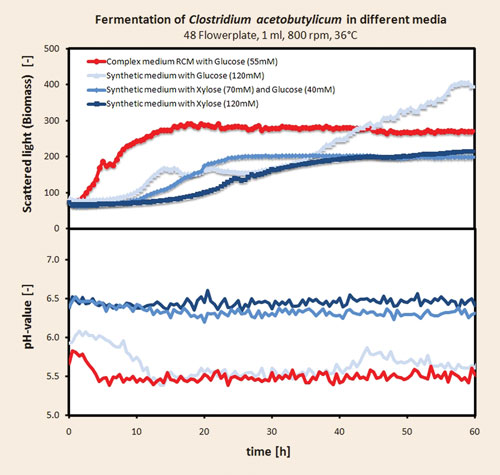
Figure 3. Results from anaerobic fermentations with Clostridium acetobutylicum at 1 mL scale with online monitoring
Outlook
The online measurement data provided by BioLector allows researchers in systems and synthetic biology to easily compare growth rates and growth phases of different clones or different media. As a result, it is now possible to screen whole libraries of anaerobic clones and evaluate their growth performance on different substrates.
Frank Kensy ([email protected]) is managing director, and Oliver Born is project engineer at m2p-labs. Burkhard Otte is a Ph.D. student at the Fraunhofer Institute of Molecular Biology and Applied Ecology (IME). Stefan Jennewein ([email protected]) is group leader for Industrial Biotechnology at the Fraunhofer IME.