
Senior Manager of Strategic Marketing, Sciex
If you are fit, it is easier to master life,” said Johanna Quaas, a 91-year-old woman from Germany. Johanna was speaking as an elite athlete. She started competing in her youth, went on a hiatus after World War II (when she took up handball), and started competing again when she turned 57. Eventually, she was named the oldest gymnast in the world by Guinness World Records.
Johanna recently noted that she exercises daily and practices gymnastics twice a week.1 “I do gymnastics to avoid being susceptible to falls,” she added. “My face is old, but my heart is young.”
Joanna sets a standard for fitness and health that many of us, young and old, would like to meet. And even the young and fit and healthy among us would like to go on emulating Joanna into their elder years.
Many of us in the United States are aware that our population, like populations elsewhere, is “super aging.” As societies age and demographics shift worldwide, we are challenged not only to cope with longer lifespans, but also to maximize our “healthspans.” A person’s healthspan is the part of their life during which they are generally in good health. Many would argue that our ability to extend a person’s lifespan is immaterial if we are unable to also extend their healthspan.
As people approach their elder years, many become more vulnerable to disease. The increased burden on our healthcare systems cannot be met by a shrinking younger population. To tackle this issue, researchers and governments are looking to preventive and personalized health solutions. Besides addressing important public health challenges, these solutions will help people stay youthful, aging with dignity and grace, and keeping their dreams alive. Imagine if you, too, could climb Mount Everest when you are 70 years old, or 75, or even 80—like Yuichiro Miura, a Japanese alpinist who at 70 became the oldest person to reach the summit, and who did so again at 80, breaking his own record.2
Preventive and personalized health solutions that can enhance lifespan and healthspan are being developed with advanced technologies such as those in the field of mass spectrometry. These technologies are empowering life scientists to discover how the mechanisms of aging lead to disease, and what we can do about it.
For example, life scientists at the Buck Institute, the first independent biomedical research institution in the world dedicated to the study of aging, are using mass spectrometry to uncover proteomic patterns that could help explain how aging works. One of these scientists, Birgit Schilling, PhD, an associate professor at the Buck Institute, recently assembled a proteomic atlas that points to biomarkers of aging.3
We all know someone who naturally looks younger than their chronological age—but what accounts for their more youthful “biological age”? To help answer this question, Schilling and her colleagues continue to sift through proteomic patterns. This work, the scientists hope, will uncover cell-level differences between individuals who “age well” and those who don’t.
Stressed-out cells lead to signs of aging
Schilling’s focus is on cellular senescence, a complex stress response that causes cells to irreversibly stop proliferating and to start secreting a range of specific proteins associated with senescence. By secreting these proteins, the cell is said to have developed a senescence-associated secretory phenotype (SASP). Although cells with SASP are generally transient in young healthy tissues and individuals, older tissues and individuals tend to have more senescent cells. This effectively chronic SASP has been known or suspected to be a key driver for many pathological hallmarks associated with aging, such as chronic inflammation (implicated in arthritis), tumorigenesis (in cancer), and impaired stem cell renewal (meaning longer tissue healing times).
Using advanced tandem mass spectrometry technologies, such as the highly sensitive quadrupole time-of-flight Sciex TripleTOF 6600 and 5600 instruments, Schilling and colleagues performed a proteomic analysis of the proteins secreted by different kinds of cells with a SASP phenotype. They discovered that contrary to popular opinion, the SASP—which had typically been assessed by analyzing a few dozen secreted proteins—was not a single phenotype but rather a range of very diverse phenotypes.
“We were very excited to discover that SASP was not just one phenotype but instead a range of highly complex and dynamic phenotypes that varied depending on senescence inducer and cell type,” declares Schilling. “This led us to map thousands of SASP proteins to create a proteomic database, the SASP Atlas, which can now be used to help identify biomarkers of aging and senescence-driven diseases, including diseases we typically associate with aging, such as Alzheimer’s disease and cancer.
“To map the atlas, we used an innovative mass spectrometry workflow that allowed us to quickly scan a large number of samples with high precision and excellent protein coverage. The efficient and streamlined workflow centered on a data-independent method called SWATH Acquisition, which scanned samples using variable precursor isolation windows.” (Here, SWATH pertains to SWATH-MS, that is, Sequential Windowed Acquisition of All THeoretical Mass Spectra.)
Different stressors cause different senescence phenotypes
Soluble and exosomal proteins were analyzed from multiple cell types, namely primary human lung fibroblasts and renal cortical epithelial cells, responding to a variety of stressors (or senescence inducers), including ionizing X-ray irradiation, inducible RAS overexpression, and atazanavir treatment. The cultured cells took 1–2 weeks after exposure to the stressor to develop a SASP. Nonsenescent (quiescent) cells were used as negative controls.
By comparing the proteins secreted by the SASP cells against those secreted by the quiescent cells, Schilling and colleagues isolated and identified SASP proteins (or factors). These were the proteins that were secreted with at least a 1.5-fold significant difference between the two sets of cells. Even though a 1.5-fold difference is not all that large, the precision of the MS methodology and SWATH Acquisition means that even so small a difference can be and is accurately quantified, and thus it can be and is considered significant.
In fact, most of the proteins measured were secreted at much higher levels by the senescent cells than by the nonsenescent cells, including nearly all the known SASP proteins. Known SASP proteins include cytokines, immune modulators, growth factors, and certain enzymes. The SASP Atlas comprises a secretome database of soluble proteins and proteins from exosomes.
The different types of stressor resulted in relatively different and discrete SASPs in the same cell type, in this case, the cultured fibroblasts originally obtained from humans. There was, however, an overlap of around 150 proteins, which suggested that there may be some core biological pathways involved that are common to all SASPs.
To elucidate whether this was the case, Schilling and colleagues used artificial intelligence (AI) to perform an unsupervised machine learning analysis of the soluble proteins in the secretome database. This clustering analysis identified three primary clusters of core SASP proteins, with one cluster strikingly consisting of only three proteins, chemokine C-X-C motif ligand 1 (CXCL1), matrix metallopeptidase 1 (MMP1), and stanniocalcin 1 (STC1). These three proteins were highly represented in all the SASPs, regardless of stressor type, suggesting that they may serve as surrogate markers (or candidate biomarkers) of SASP and senescence.
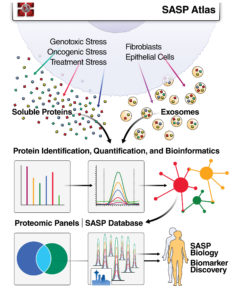
Although the various stressor types resulted in distinct signature SASPs, the generation of the SASP Atlas can inform and facilitate the discovery of biomarkers for aging and aging-related diseases. A recent biomarker study identified 217 proteins that are significantly associated with age in human plasma.4 Of these, 20 proteins were present in the originally defined single SASP.
Remarkably, 92 of the known and now newly discovered SASP proteins in the SASP Atlas are also known biomarkers of aging in human plasma. Moreover, these aging biomarkers include 39 of the core SASP proteins identified using the clustering analysis. Thus, known biomarkers of aging that can be detected in human plasma include numerous SASP factors. The enrichment of aging and age-related disease biomarkers in the secretomes of senescent cells supports their link to a wide spectrum of age-associated conditions.
Aging and SASP biomarkers
This analysis of the secretomes of senescent cells reveals that there is much more to learn about how we age, both at a cellular level and as a whole organism. As we progress toward identifying more biomarkers of aging and age-related disease, we can look toward developing companion diagnostics to identify which individuals would most benefit from interventions (and determine when interventions should occur) to maximize their healthspan and lifespan.
“Through the SASP Atlas—our comprehensive proteomic database of soluble proteins and exosomal cargo SASP factors originating from multiple senescence inducers and cell types—researchers can now identify several candidate biomarkers of cellular senescence that overlap with aging markers in human plasma,” concludes Schilling. This takes us one giant leap forward toward personalized health, precision medicine, and healthier aging.
Disclaimer
The Sciex clinical diagnostic portfolio is For In Vitro Diagnostic Use. Rx Only. Product(s) not available in all countries. For information on availability, please contact your local sales representative or refer to https://sciex.com/diagnostics. All other products are For Research Use Only. Not for use in Diagnostic Procedures.
Trademarks and/or registered trademarks mentioned herein are the property of AB Sciex Pte. Ltd. or their respective owners in the United States and/or certain other countries.
© 2020 DH Tech. Dev. Pte. Ltd. RUO-MKT-19-11286-A
References
- Payne M. ‘My face is old but my heart is young’: World’s oldest gymnast still has moves at 91. The Independent. Published July 3, 2017. Accessed March 2020.
- Henderson B. Meet Yuichiro Miura, the man planning to conquer Everest at 90. The Telegraph. Published January 1, 2016. Accessed March 2020.
- Basisty N, Kale A, Jeon OH, et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLOS Biol. 2020; 18(1): e3000599.
- Tanaka T, Biancotto A, Moaddel R, et al. Plasma proteomic signature of age in healthy humans. Aging Cell 2018; 17: e12799.
Tom Knapman, PhD, is senior manager of strategic marketing, brand and creative, at Sciex, a Danaher operating company and a global leader in the precise quantification of molecules.

