November 1, 2009 (Vol. 29, No. 19)
Product Improvements Have Expanded Disposables’ Scope and Overcome Limitations
In recent years, GEN has reported on single-use bioprocess bags, connectors, sensors, and tubing many times. The benefits of disposables are, by now, well known and do not require repeating. An early knock on disposables was their limited volume, but the debut of 2,000 L bioprocess containers, accompanied by dramatic improvements in protein titers from cell cultures, dramatically expanded the scope of disposables. Yet, Sartorius Stedim Biotech estimates that single-use equipment has penetrated less than 20% of its potential biomanufacturing market.
One GEN reader, T&C Stainless CFO Todd J. Cook, takes issue with the single-use mania, citing environmental and cost issues and arguing—with some justification—that reporting on disposables paints an unduly rosy picture. “I have watched the growth of single-use systems from the beginning, and I do realize they have their place. Stainless does cost more in the startup phase of a [biopharmaceutical] product, but it must be less costly during manufacturing due to the ongoing cost of bags, and their subsequent disposal as hazardous waste.”
Cook recognizes the need for flexibility in one-off processes, in multiproduct facilities, or early in development when the cost of investing in stainless equipment may not be justified. But stainless steel is preferred in single-product plants, particularly immediately post-approval. “I know of stainless systems that have been in use for 20 years or more. The cost of single-use systems during that period would be vast.”
But proponents of single-use equipment point out that bioreactors are just one variable in the cost equation. Cleaning materials used to sanitize steel tanks, including high-purity water, are expensive and carry a significant carbon footprint and time investment that rapidly adds up. The cost of electricity that powers a facility during downtime adds to the environmental impact per batch. Then there are the substantial human resource costs associated with documentation and cleaning/validation operations.
Plastics have environmental consequences as well. Plastics manufacture carries a significant carbon footprint, uses nonrenewable feedstocks, and involves environmentally unfriendly disposal. “I was in the plastics industry for 15 years before my present position,” Cook told GEN, “and have seen the cost associated with producing plastics from crude oil, the production of the product itself, and the cost of disposal. Recycling [of bio-bags] is out; landfills are not an option due to the fact that the bags are biohazards, and even if they could be put into a landfill they never break down. That leaves incineration, which is costly and not environmentally friendly.”
Single-use process bags provide fewer paths for problem recovery, observes George Moyer, director of business development at Broadley-James. For example, if a 1,000 L stainless steel CHO culture foams and fouls an exhaust filter, it is possible to isolate or change the filter. “With a single-use bioreactor, if you foul a filter and you don’t have a replacement read to uncap and install, the game is over. The run is finished.”
Another potential issue arises from contaminated batches, and how to dispose of the culture. “With steel tanks you press the ‘auto-sterilize’ button and run the fluid down the drain,” Moyer says. “Disposables require the use of a chemical disinfectant, which may or may not be effective.”

Sartorius Stedim Biotech’s Flexboy® bags provide a single-use disposable alternative to traditional glass and plastic.
A Matter of Perspective
In its sixth annual report on trends in single-use bioprocessing, BioPlan Associates put some of the concerns over single-use equipment into perspective. Author Eric Langer notes that the question for most bioprocessors is not whether single-use equipment will be deployed, but where and how (and by implication, when). According to BioPlan’s survey, “concerns over adoption are rapidly becoming less strategic and more operational and commercial,” and “objections to disposables have declined both in quantity and importance.”
For example, worry over leachables and extractables among the 443 production executives surveyed declined from 16.6% in 2008 to 10% in 2009. Moreover, these executives were more concerned with cost-effectiveness than whether single-use equipment would serve their purposes. Larger companies were less likely to be interested in increasing their disposables usage, a fact that reflects the number-one economic issue at play here: investment in capital equipment.
Seventy-three percent of respondents at large companies cited “existing equipment” as a hindrance to adopting disposables, compared with just under half of executives at mid-sized firms. Even so, this objection decreased, from 19% of respondents last year to 14% in 2009. And 13% overall agreed with the statement that “disposables are expensive.” Interestingly, contract manufacturers, the group often cited as prime customers, were 20 percentage points more likely than sponsors to name cost as a key impediment to greater use of disposables.
Michiel Ultee, Ph.D., who heads process science efforts at Laureate Pharma, concurs with BioPlan’s findings, at least on the subject of capital investment. “In my experience, stainless steel bioreactors are favored over disposables whenever a company has already invested in stainless steel tanks, and this equipment is validated and running well. This is especially true for repeated runs of the same process.”
Process volume is another area where manufacturers often have little choice but to specify stainless steel. The largest disposable bioreactor currently available, from Xcellerex, holds 2,000 liters. “And bag costs and handling become an issue when the bags get really large.”

Laureate Pharma is focused on two segments of the biopharmaceutical industry–monoclonal antibodies and recombinant protein products.
Middle Ground
One could summarize all objections to disposables with one question: Is product produced in a plastic container of the same quality as that manufactured in more traditional stainless steel and glass bioreactors? Biotechs and contract manufacturers, whose very existence depends on consistency, have embraced disposables but temper their enthusiasm with due diligence.
Mid-sized CMO Avid Bioservices has “always” used disposables for storage and buffer prep, says Rich Richieri, senior vice president of manufacturing, but the company is taking its time in validating disposable bioreactors. Avid has been particularly focused on maintaining traditional bioreactor geometry and making sure that the cells were mixed and sparged in an equivalent manner. So far everything checks out.
Last November, Avid began comparing batches run in 1,000 L single-use bioreactors with product produced in its three stainless steel tanks. “We haven’t noticed any differences so far through typical lot-release testing,” Richieri notes, “but our next campaign will look much more deeply into extractables.” Leachables and extractables from bags have been on the worry lists of every biomanufacturer, bag vendor, and all major regulatory agencies.
As a mid-sized manufacturer, Avid is perhaps emblematic of that middle ground in process volume, where battles between steel and plastic are ongoing. Jeff Jackson, sales director at Bosch Packaging, recognizes this fuzzy divide.
According to Jackson, Bosch has introduced the industry’s first filling line with fully disposable product contact surfaces. The company also sells traditional steel reusable systems, though. “There comes a point—and I can’t say specifically where that is—where costs associated with acquiring and setting up a dedicated filling machine become burdensome.”
The amortization of those costs, he says, works better for a run of one million vials vs. a 20,000-vial run. “If you’re producing a large number of doses of a single product, there is no chance of cross-contamination and you only need one dedicated set of hardware, or perhaps one backup as well. You wouldn’t have a huge investment in dedicated contact parts.”
Recycling Bioreactors
It has been argued that the purchase of used equipment could make steel bioreactors even more cost-effective, over lifetime use, than disposable bags, while amortizing the environmental impact of producing the steel and fabricating it into a GMP-worthy vessel even more favorably. Generally, the arguments for used equipment can be quite compelling: used equipment is widely available, is significantly less expensive than brand-new, and the environmental hit for its manufacture has already been taken.
Moyer believes this is a gross oversimplification. While quality used products can be had for a song in non-GMP industries, biomanufacturers are extremely wary of purchasing used equipment with a high product contact profile for making drugs. On the most basic level, a bioreactor must fit a manufacturing suite and have specific utilities serving it. Knocking down walls, raising ceilings, and rearranging the plumbing are themselves capital expenditures.
Reliability is an even more serious issue. “By the time a company goes under or otherwise disposes of capital equipment, there is usually no one around who can find the documentation,” Moyer says. Under pressure to close the facility and get everything valuable out the door, large steel tanks would likely be dismantled by low-level technicians or simply get torn out. Parts get left behind or mishandled, creating a nightmare for reassembly. “The longer it sits, the more likely it will be sold by the pound,” he adds. “It’s difficult to resell this type of equipment to a GMP industry.”
End-users are loath to spend precious resources reconstructing and recommissioning a complex piece of equipment. Moyer notes the psychological factors that come into play: Even if a bioreactor is returned to apparent working order, the manager who decided to purchase it would be on the hot seat for many months for any problem that arises.
In short, it is hard to find a discouraging word regarding disposables that actually sticks. The marketplace is vibrant and expanding through start-ups, partnerships, and, paradoxically, the acquisition of single-use technologies and entire companies by vendors whose reputations were built in glass and stainless steel bioreactors.
Despite raising numerous problems, Moyer is an unabashed convert to disposables. “I’ve worked with stainless steel tanks for a long time, but now my eyes have been opened to disposables. It’s a significant change, but even large, risk-averse companies have jumped on the bandwagon. There’s no doubt that any biomanufacturer operating below a couple of thousand liters should consider disposables.”
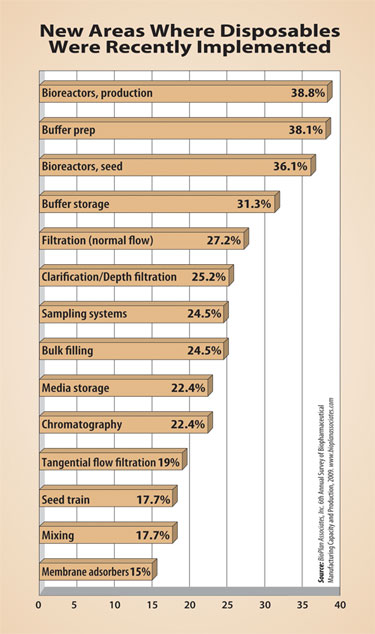
New areas where disposables were recently implemented
As Bags Go, So Go Sensors
As disposable bags and containers have become more popular, so have single-use sensors. For example, deploying a reusable sensor makes little sense in a presterilized single-use bioreactor system, since its deployment involves breaking the sterile barrier.
“Insertion of sensors requires an aseptic environment and is disruptive and difficult to maintain in a manufacturing environment,” observes Juliette Schick, Ph.D., president of SciLog.
Jim Furey, president of PendoTech, concurs. “It comes down to whether you need sterility or not. There is a risk attaching an autoclaved transducer to a presterilized assembly. You need to do so through an aseptic connection, and in any event stainless steel pressure transducers have a limited number of autoclave cycles.” One drawback of single-use sensors, however, is that while users can check their performance, the devices cannot be calibrated in the conventional sense while the sensor resides inside a sterile bag.

SciLog’s SciPure 200 utilizes a single-use fluid manifold.



