November 1, 2007 (Vol. 27, No. 19)
Hong Gao
Stephanie Thompson
Romain Verollet
Antonin Duval
Metallic and Ceramic Bead-beating Technology Is Especially Adept with Small-size Tissues
Tissue homogenization is a critical process for pharmacological analysis, especially when using a LC/MS/MS system. The ease and efficiency of homogenization, reduced cross-contamination, analyte recovery, and stability are important factors that affect data quality and high-throughput productivity.
Traditionally, tissue homogenization involves a mechanical shearing force, for example, blending or grinding. These processes produce high temperatures that may cause analytes in the matrix to become unstable. In addition, some small tissues such as rat or mouse lymph nodes and tumors are often difficult to handle with mechanical blenders. Such tiny pieces often must be manually grinded, which is time and labor intensive.
Bertin Technologies (www.bertin.fr) designed a new homogenizer featuring a bead-beating technology. This system, Precellys-24 (Figures 1 and 2), allows homogenization of a wide range of biological samples from soft to hard, and even elastic, and specializes in small-size tissues. This adaptability is made possible by adjusting the type of beads (ceramic or metal) and the speed of the homogenizing motion.
Precellys-24 uses a figure-eight motion to rapidly gyrate beads to grind up to 24 samples at one time. Moreover, each of the 24 sample tubes are single-use, which reduces the risk of cross-contamination between trials and facilitiates storage.
In a recent application, Precellys-24 was used to homogenize rat brain, gonad, heart, kidney, lung, spleen, lymph node, and tumor tissue samples for pharmacology and ADME studies.
Figure 1
Sample Preparation
All tissue samples weighed less than 1 gram, with weight depending on the tissue hardness. Tissue samples were placed into the Precellys-24 2 mL tube in a 1:2 ratio of tissue to buffer (de-ion water). The sample tubes were then placed in the Precellys-24 for homogenization.
An aliquot of 100 mg homogenate of each sample was then placed into a 96-well plate. Blank matrix homogenate was also prepared for calibration standards, quality control samples, and matrix blanks in the sample plate.
To each 100 mg homogenate, 400 µL acetonitrile with internal standard was added for analyte extraction. After centrifuge at 3,000 rpm for 20 minutes, the supernatant was injected onto a LC/MS/MS system for sample analysis.
An Agilent(www.agilent.com) 1100 HPLC pump was connected to Applied Biosystems’(www.appliedbiosystems.com) API 4000 LC/MS/MS for sample analysis. Waters’ (www.waters.com) XTerra MS C18 (5 µm, 2.1 x 50 mm) analytical column was used for the chromatography. A one-minute linear gradient of acetonitrile/10 mM ammonium acetate was applied for the analyte elution. Flow rate was 700 µL/min. Positive ions were acquired in the multiple reaction monitoring mode under APCI ion source. Nebulizer temperature was set at 400°C.

Figure 2
Results and Discussion
The efficiency of tissue homogenization was improved 5- to 20-fold using Precellys-24 compared to blending or grinding. In one run, 24 samples were homogenized within one minute using Precellys-24. Conversely, it took one minute for each individual sample to be prepared when using manual blending or grinding. For analyte recovery, there was no difference between the two methods
The assay was also reproducible within analytical runs. All types of tissue sample analysis demonstrated linearity, accuracy, and precision (Figure 3). Metallic beads (Protocol 1 and 2) worked well for all types of tissues, but homogenization using ceramic beads (Protocol 3) required more cycles for harder tissues.
Precellys-24 is a reliable homogenization system for a wide range of small-size tissues. The instrument performs best with tissues weighing around 20–50 mg. Studies have shown that Precellys-24 excels at handling rat lymph node and tumor samples. Sample size, however, should be adjusted to less than 0.5 g for harder tissues such as tumors, kidney, and heart.
Efficiency will be increased if the tissue samples can be directly collected into the Precellys-24 2 mL tubes. In addition, metallic beads can be used for all types of tissue samples with minor adjustments to speed or cycle number. Most importantly, when the protocol is set up and validated, sample preparation is always the same.
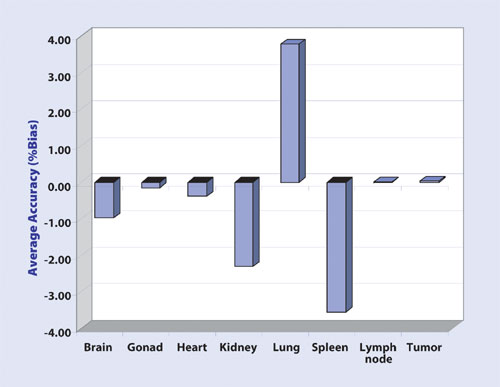
Figure 3
Hong Gao ([email protected];
617-444-6415) is senior research
scientist, and Stephanie Thompson
is research associate at Vertex Pharmaceuticals. Web: www.vrtx.com. Romain Verollet is biotechnology product manager, and Antonin Duval is biotech business manager at Bertin Technologies. Web: www.bertin.fr.