October 1, 2017 (Vol. 37, No. 17)
Automated Filling Operations Cut Batch Processing Time in Half
Cell banks are a vital part of research and drug discovery, biologics, or cell-therapy manufacturing processes. Generating cell banks traditionally involves uncapping hundreds of vials for each batch, pipetting cell suspensions into cryovials as quickly as possible, and then recapping each vial. Performing these tasks manually is time consuming, increases the possibility of microbial contamination, and the repetitive movements puts scientists at risk of occupational repetitive strain injury.
Exposure time to cryoprotectants must be as short as possible to maintain optimum cell quality and minimize cytotoxic effects. Vial-to-vial consistency is also important and can become less precise as scientists get fatigued during the prolonged process time. This often means the number of vials in a cell bank is limited to a few hundred using manual processing.
Regulatory authorities require evidence that processing frozen vials of reference standards and cell banks is both traceable and reproducible, so each batch must undergo QC testing before release to other sites. Therefore, multiple batches each containing a few hundred cryovials are more expensive to produce in terms of labor, testing, consumables, and reagents than a single batch containing several hundred cryovials or more.
To overcome these quality, consistency, cost, and occupational injury issues, liquid-handling systems have been developed that can automate dispensing into multiple vials in parallel. This makes it possible to create larger banks in shorter periods of time and helps ensure consistency across the batch, as well as the traceability required by the regulatory authorities. The drawback with using these systems for cell-banking applications is the pipettes for aspirating and dispensing frequently cause shear damage that affects cell viability.
Alternative dispensing systems have been introduced, which use peristaltic pumps to fill vials. This reduces the shear damage and maintains viability before and after freezing. However, these systems do not automate opening and closure of the vial so do not eliminate the most time-consuming activity. Nor do they address the occupational safety or potential contamination issues surrounding manual processing. So, there remains the need for a system that automates opening and closure of the vial in order vial to speed up processing time and protect scientist from repetitive movements, and that can fill vials accurately and consistently with minimal cell damage.
Automated Cryovial Processing Platform
The fill-it system (Sartorius Stedim Biotech) is a bench-top automated cryovial processing system (Figure 1). The system automatically uncaps, fills, and recaps screw-cap cryovials held in racks of 24, 48, or 96, and is compatible with cryovials from most leading manufacturers. Use of the system speeds up cell bank creation and can produce a given batch size in half the time it would take a scientist to process the same number of cryovials manually. This allows the generation of larger batch sizes while reducing QC costs and providing scientists with a safer production method.
To minimize the risk of contamination, the fill-it system is compact enough to be used in a standard laboratory cell culture cabinet and utilizes a sterile, single-use tube set for each cell bank batch. Dispensing is performed via a gentle peristaltic pump action that minimizes cell damage and helps maintain cell viability.
To demonstrate the suitability of fill-it for cell-banking applications, the system was evaluated at a contract manufacturing and development organization (CDMO) to measure key performance parameters of dispensing accuracy, processing time, and cell quality.

Figure 1. The fill-it automated cryovial processing system from Sartorius Stedim Biotech.
Dispensing Accuracy
The dispense accuracy of an automated filling system is vital for ensuring vial-to-vial consistency in a cell bank. To assess this, the fill-it system was calibrated for a 1.5-mL dispense volume and 21 racks of 24 cryovials were processed sequentially. For comparison, cryovials were also filled manually by two different scientists using a pipette also calibrated for a 1.5-mL dispense volume. Pre-weighed tubes were removed from across the 21 racks and re-weighed to gravimetrically assess the volume dispensed. Tubes prepared manually were also weighed pre- and post-dispense. The mean, standard deviation, and %CV was calculated for both automated and manual dispensing.
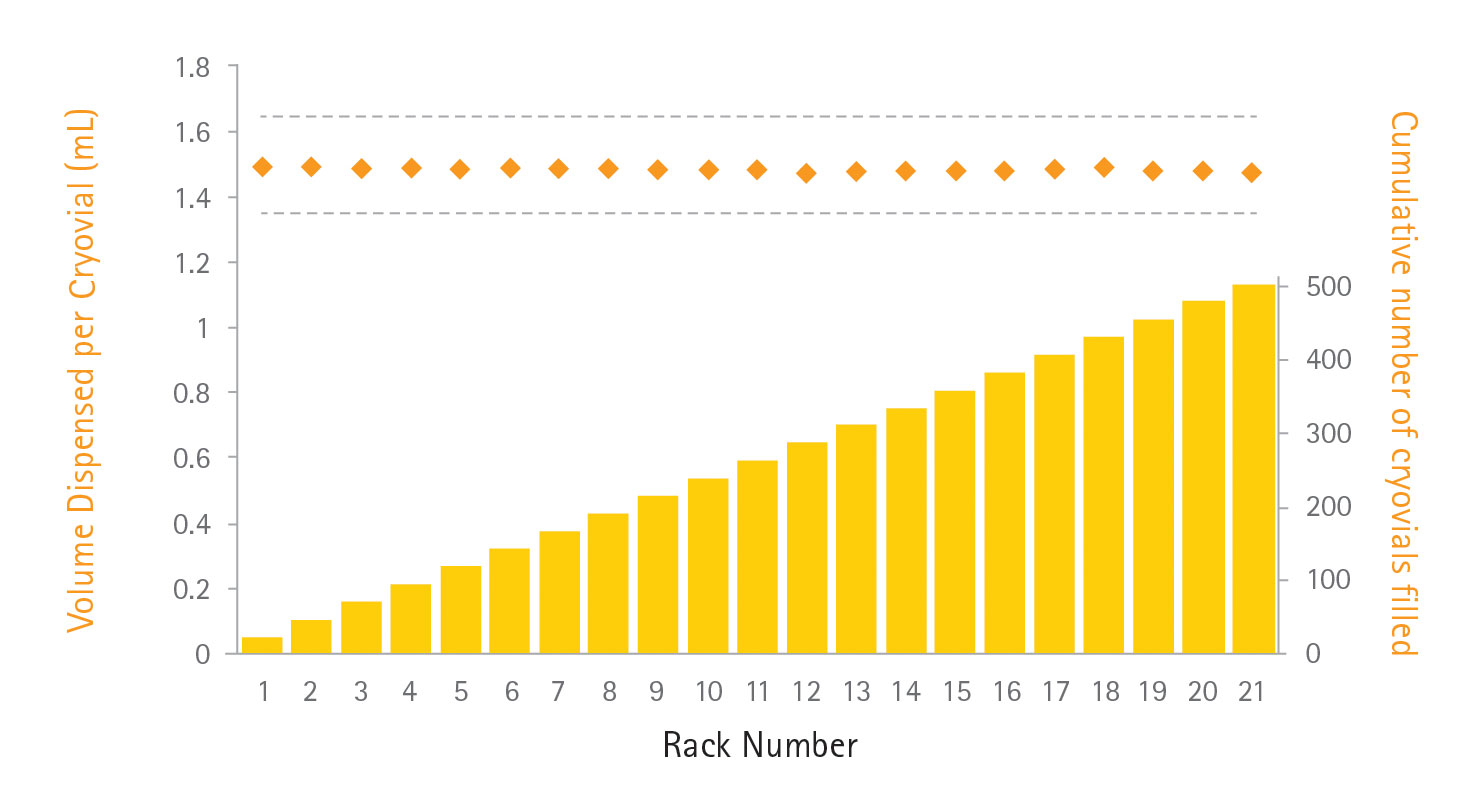
Data summarized in Figure 2 illustrate the consistency of automated dispensing across the 21-rack run with a %CV of 1.1%. In comparison, cryovials filled manually have %CV of 1.3% and 3.3%, demonstrating how using multiple operators can introduce variation into cell-banking operations. The accuracy of dispensing was consistent with that achieved with manual dispensing and indicates the fill-it system will perform consistent vial-to-vial filling with an accuracy and precision equivalent to, if not better than, manual dispensing. As Figure 3 shows, this could be maintained across the full range of 21 racks (504 cryovials) dispensed, ensuring good intra- and inter-rack consistency within the cell bank.
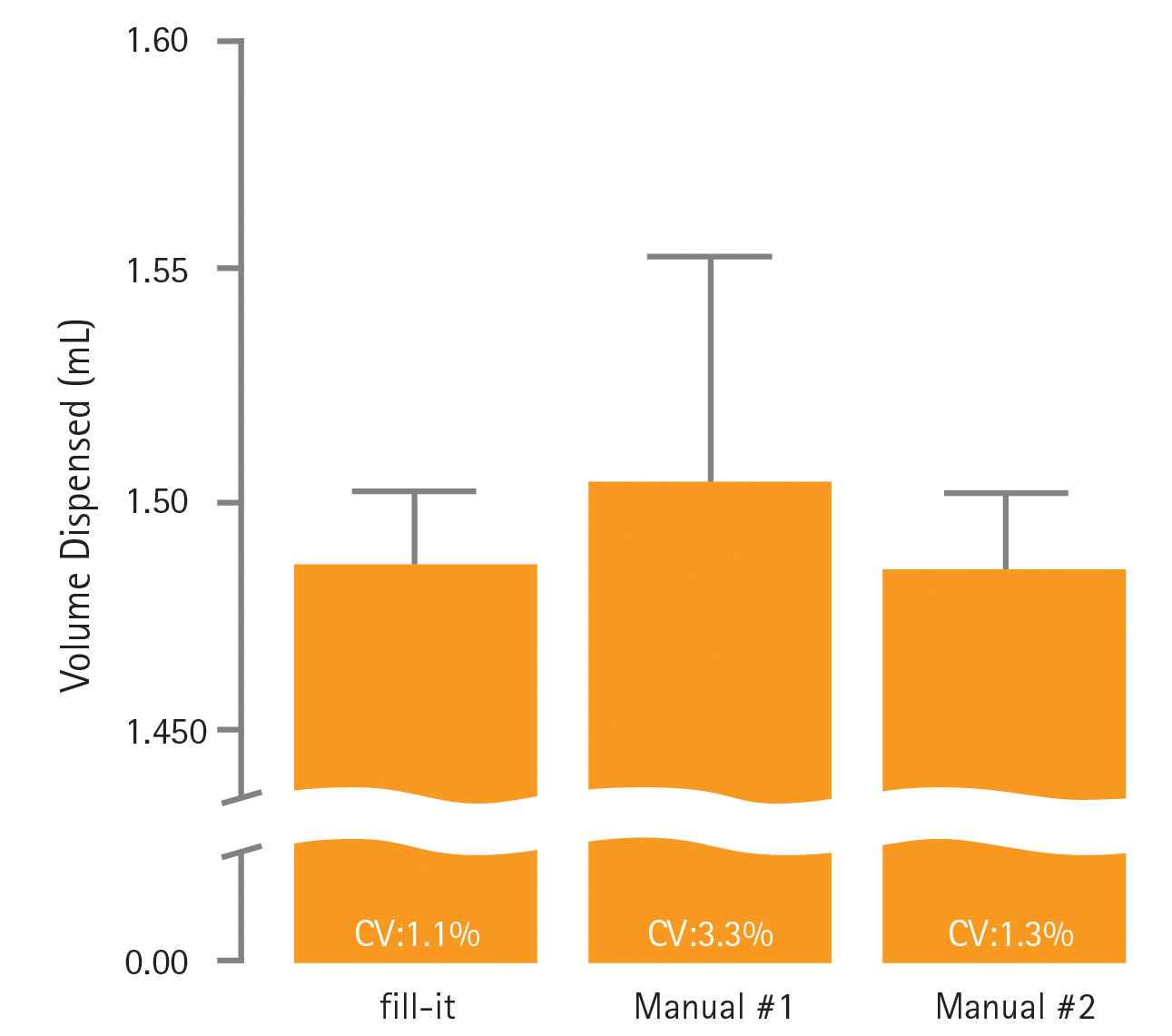
Figure 2. Accuracy and consistency of automated and manual dispensing into cryovials. Data shown are mean ± SD.
Figure 3. Accuracy and consistency of automated dispensing into cryovials across a 21-rack (504 cryovial) cell bank. Data in orange show the mean volume dispensed per cryovial per rack, grey lines indicate the ±10% accuracy claim made for the sterile single-use tube sets, and yellow bars denote the total number of cryovials filled.
Processing Time
The time taken to process batches of 200 and 500 cryovials using the fill-it and manual processing was measured and results shown in Figure 4. In both cases, the automated system provided a significantly faster processing time, such that a 500-vial cell bank could be created by fill-it in less time than it would take to create a 200-vial cell bank manually.
This ability to quickly process large cell banks can lead to cost savings by reducing the frequency of creating additional cell banks. Each cell bank generated must undergo QC including in vitro cell age and formal equivalence studies—as well as regulatory submissions—therefore, larger cell banks reduce the number of QC studies and submissions required.
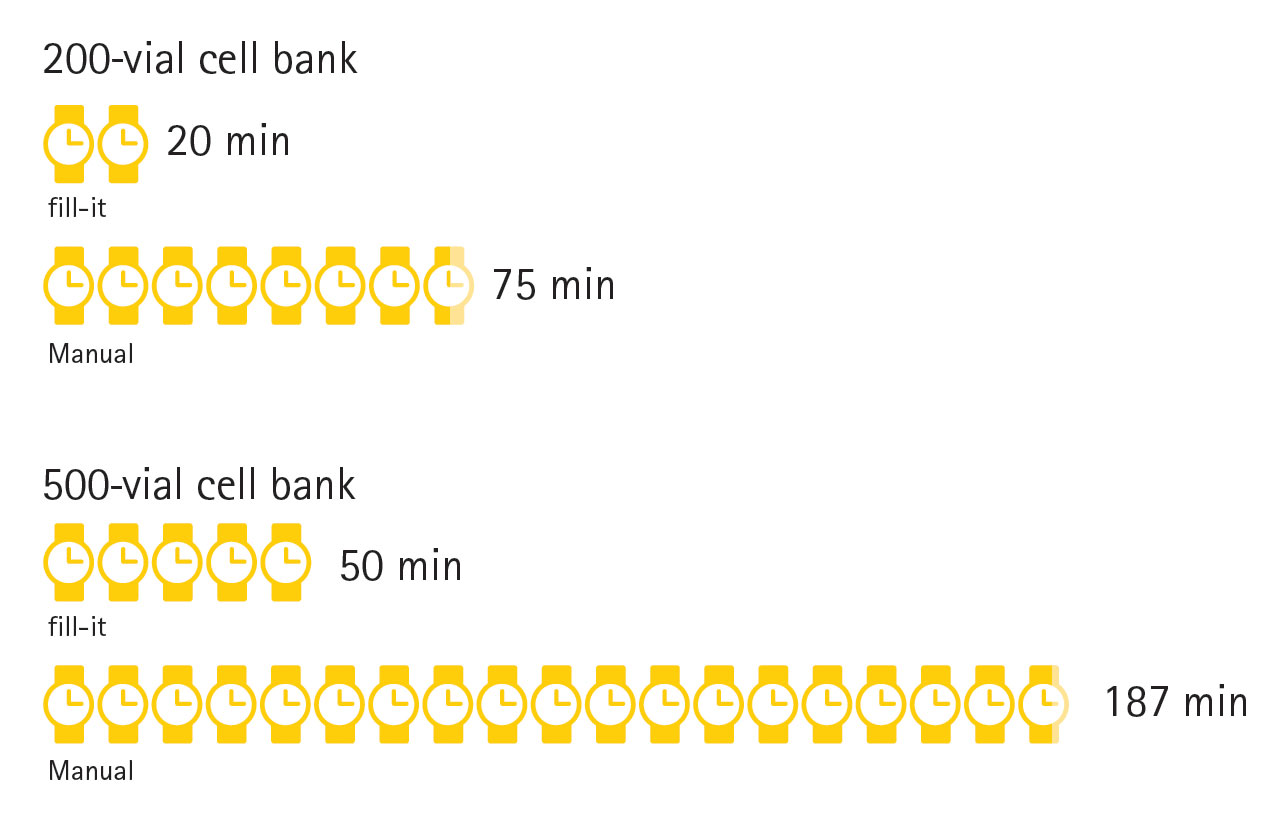
Figure 4. Comparison of time required to produce a master cell bank using an automated system versus manual pipetting methods.
Cell Quality
To demonstrate that the fill-it system can help maintain sterility and cell viability, CHOK1SV cells were revived from a master cell bank and cultured in shake flasks using chemically defined animal-component-free media. The cells were pooled, centrifuged and re-suspended at 1.0×107 cells/mL in media containing DMSO as a cryoprotectant before dispensing into 500 cryovials using a fill-it system in a Class II microbiological safety cabinet. Vials were then transferred to a controlled-rate freezer for cryopreservation.
Vials from the cell bank were tested for sterility and mycoplasma at critical stages, and environmental monitoring was performed for viable/non-viable particles according to current European, United States and Japanese Pharmacopeia methods. There was no detection of microbial or mycoplasma growth in the cell bank prepared by fill-it.
Following 48 hours of storage at -130°C, 25 sample vials from across the 500-vial cell bank were revived by rapid warming and seeding into shake flasks containing media and passaged over a period of 19 days to assess viability and homogeneity of propagation. Cell numbers and cell viability were measured at revival and during the first passage at 72 hours post-revival. As a comparison, a vial of CHOK1SV cells was also revived from a manually prepared master cell bank and assessed at revival and at 72 hours. Cell numbers and viability were measured upon revival and 72 hours after being passaged.
The data in Figure 5 show the range of cell counts and cell viabilities measured for cultures generated from the vials prepared with fill-it. At revival, the average viable cell count and cell viability was 1.2×106 cells/ml and 95%, respectively. After 72 hours, this had risen to 2.3×106 cells/ml and 96%. By comparison, the vial revived from the manually prepared cell banks had a cell count and viability of 1.1×106 cells/ml and 93%, respectively, at revival, and 1.8×106 cells/mL and 92%, respectively, after 72 hours.
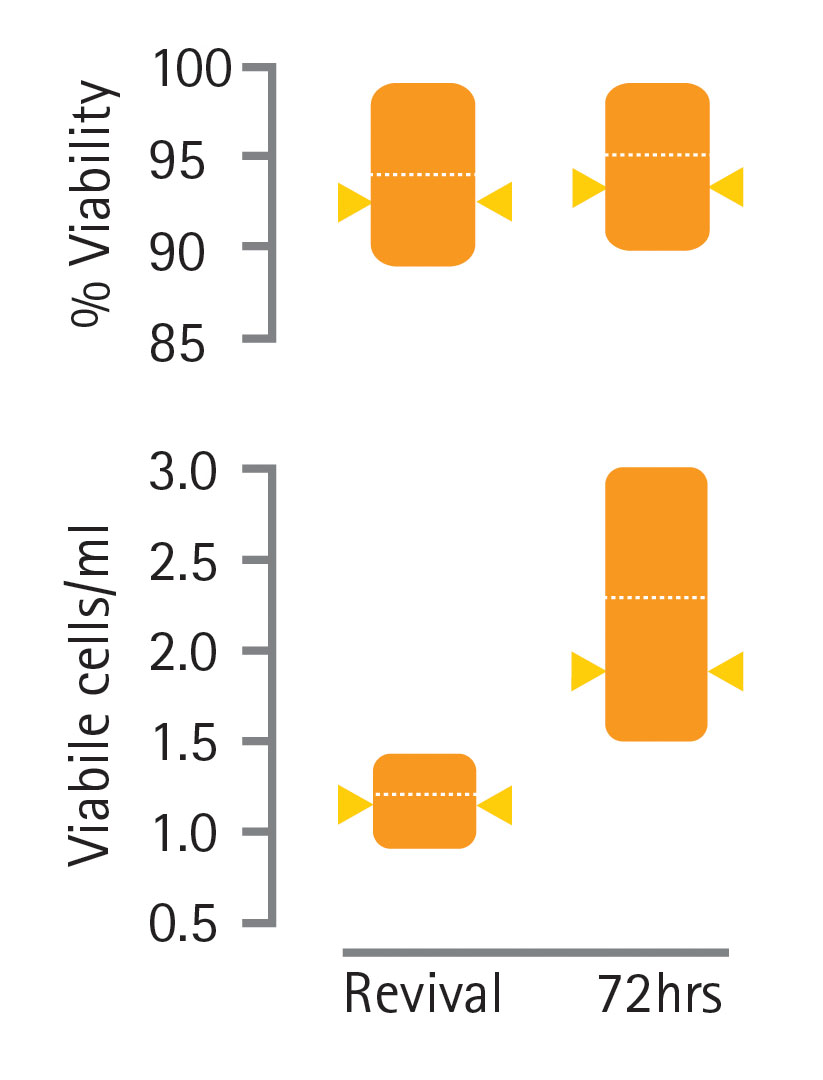
Figure 5. Viable cell counts and cell-viability measurements of CHOK1SV cells revived from a cell bank prepared using fill-it. Orange bars illustrate the range of viable cell counts and percentage viabilities from 25 vials. The dashed white line signifies the mean. Yellow arrowheads show the values obtained from a vial of CHOK1SV cells revived from a manually prepared cell bank, for comparison.
Conclusion
The accuracy and precision of automated dispensing using fill-it is equivalent, or better than, manual dispensing, yet allows for the preparation of a cell bank of 500 cryovials in a third of the time it would take to prepare the same number of vials manually. A master cell bank produced using fill-it was negative for microbial contaminants, and vials from this cell bank achieved viable cell counts at revival and sub-culture at rates comparable to cells drawn from a manually prepared cell bank.
In summary, the fill-it automated cryovial processing system can be used to rapidly generate large numbers of cryovials. Vial-to-vial consistency is good, vials are free from contamination, and cells can be revived and propagated with the same levels of success as cell banks prepared manually. All these features make the fill-it system suitable for cost-effective GMP cell-bank preparation.
Dave Thomas, Ph.D. ([email protected]), is a product manager at Sartorius, Royston, U.K.



