May 1, 2016 (Vol. 36, No. 9)
With approximately 30 to 40 percent of all marketed prescription pharmaceuticals acting on G protein-coupled receptors (GPCRs), these receptors remain attractive investigational drug targets, even though the complexities of GPCR signaling continues to make GPCR-based drug discovery challenging.
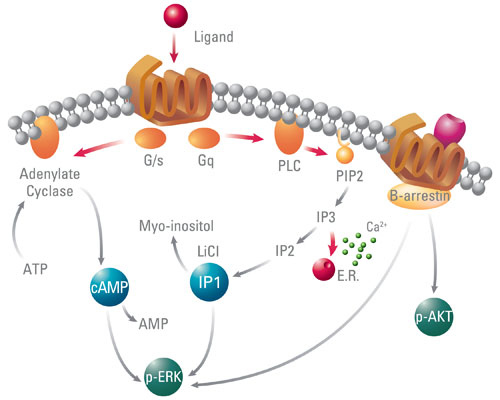
GPCRs have two principal signal transduction pathways that can be detected via the second messengers, cyclic AMP (cAMP), and phosphatidylinositol (IP), a metabolic product of IP3 (Figure 1). There are many commercial biochemical and cell-based assays that measure these second messengers but it is well established, that effectors other than cAMP and IP are involved in GPCR signaling pathways and several receptors are not optimally coupled through the cAMP and IP pathways. Therefore, it is important to assess other cellular effectors activated by GPCRs such as the extracellular signal-regulated kinase (ERK), which can be activated by both G protein and β-arrestin. Since ERK activation is an important signaling pathway associated with many physiological functions, targeting this can offer new research strategies for GPCR-based drug discovery.
In specific therapeutic areas, the need to assess endogenously expressed GPCRs in specific cellular models is key to accessing more physiological relevant conditions. This has led to many drug discovery programs utilizing cellular models including engineered cells or immortalized cell lines which overexpress GPCRs in their drug screening. However, in these studies since the availability of samples including tumor patient-derived cells is often limited and/or more complicated and expensive to investigate, the need for highly sensitive assays that use small amounts of sample and low reagent volumes is becoming increasingly important.

Figure 1. Main readouts for GPCR signaling pathway investigations, including cyclic AMP, IP1, and p-ERK.
Advanced Phospho-ERK1/2 Assay
These assay miniaturization and detection issues have led Cisbio Bioassays (www.cisbio.com) to develop the Advanced Phospho-ERK1/2 (Thr202/Tyr204) kit to measure the ERK alternative signaling pathway. This assay utilizes highly sensitive, well-established HTRF (Homogeneous Time Resolved Fluorescence) technology and can be used with any cellular model. With the assay, scientists can utilize the same HTRF technology to link their upstream (cAMP, IP) and downstream Phospho-ERK (p-ERK) GPCR signaling pathway readouts, thus using less of their rare or expensive cell samples, which can improve the cost-effectiveness of high-throughput screens, without compromising on data quality.
Proof-Of-Concept
The application of this p-ERK assay has been validated on stable cell lines, immortalized cell lines, cancer cells, and a tumor patient-derived cell type stimulated with appropriate agonists, for receptor-mediated downstream ERK1/2 phosphorylation.
For example, the assay was assessed in proof-of-concept experiments using the stable HT29 colon adenocarcinoma cell line (ATCC HTB-38, USA) and physiological relevant, patient-derived cells isolated from hepatic metastases of human colorectal cancer (J. Pannequin, IGF Montpellier, France). The HT29 cells were cultured in McCoy’s 5A + GlutaMAX (Thermo Fisher Scientific, USA) + 10% Fetal Bovine Serum FBS / FCS (Thermo Fisher Scientific, USA) + 1% Penicillin/Streptomycin (Thermo Fisher Scientific, USA) and the patient-derived cells in DMEM + GlutaMAX (Thermo Fisher Scientific, USA) + 10% Foetal Bovine Serum FBS / FCS (Thermo Fisher Scientific, USA) + 1% Penicillin/Streptomycin (Thermo Fisher Scientific, USA) media for 24 hours (37°C, 5% CO2) in black 96-well flat clear bottom microplates, (Greiner Bio one, Austria). The cells were lysed and lysate (16 µL) was dispensed in triplicate into 384-well white plates (Greiner Bio one, Austria), with reagents from the Advanced Phospho-ERK1/2 (Thr202/Tyr204) kit (Cisbio, France) and a range of doses (0.001–1000nM) of the agonist, neurotensin (PolyPeptide, France).
The assay was incubated (room temperature, 12 hours, and the HTRF signal was analyzed with a PheraSTAR FS reader (BMG Labtech, Germany). The results (Figure 2) show that with both cell types the assay is highly sensitive, producing well-defined, complete dose response curves from which EC50 values of neurotensin were easily derived. The EC50 value seen in the HT29 cells is comparable with published EC50 values in HEK-293T of 1.1 nM, showing the accuracy of the assay with recombinant cells expressing the neurotensin receptor.

Figure 2. Detection of ERK 1/2 protein phosphorylation mediated by neurotensin on HT29 cells (green) and in tumor patient derived cells (orange).
Optimizing the Assay
Many different parameters can be identified when using this HTRF p-ERK assay, from the preparation of working cells through to cell lysis, that if not carefully adhered to, can lead to sub-optimal results from drug screening or drug profiling. These include inaccurate EC50, values and assay windows that are too small for meaningful data capture. Therefore, to generate optimal results from this type of assay, there are key points that scientists should consider including in their workflow.
For example, cell densities need to be tested to either ensure an optimal signal, or determine the best compromise between conserving cell samples and detecting ERK phosphorylation. Additionally, the concentration of serum has to be assessed because the presence or long absence of serum in the cell culture medium during the plating and stimulation step may alter GPCR-mediated p-ERK detection. For example, in a serum optimization study no serum starvation and overnight serum starvation conditions both led to low signal amplitude, due to strong basal activity and resulted in different EC50 values when compared to cells starved of serum for two hours (Figure 3).
Another strategy for optimizing the HTRF p-ERK assay is to dispense compounds in the presence of the cell-culture medium the cells were originally cultured in. This is because removing media and adding fresh cell culture media causes stress to cells and the resulting high basal activity masks p-ERK detection. One final tip for accurate assay performance is to optimize the stimulation time and temperature as signal amplitude may be considerably decreased by stimulating cells for too short or too long a time.
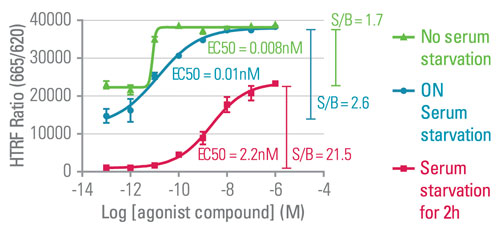
Figure 3. The effect of using a range of serum concentration conditions on the detection of p-ERK with an HTRF-based p-ERK assay.
Conclusion
The proof-of-concept study demonstrates that Cisbio’s Advanced Phospho-ERK1/2 (Thr202/Tyr204) assay is a robust, sensitive method of detecting GPCR stimulation with an agonist, not only in a stable cell line overexpressing GPCRs but also in more physiological relevant cell models with low ERK expression. These results indicate that the assay when combined with some simple cell-culture optimization steps could become a reliable tool for delivering accurate, reproducible data when cell sample size is limited and/or expensive to produce, or more in depth research into downstream GPCR signaling pathways is required.
Cisbio Bioassays