June 15, 2010 (Vol. 30, No. 12)
Increased R&D Efforts Are Overcoming Obstacles and Showing Potential
Ion channels make good drug targets—they reside on the cell surface and are fast switching mechanisms. They act like cell transistors, controlling many cell processes. There are close to 500 types of ion channels, yet many remain undiscovered. This was mainly attributed to technology restraints, however, with the recent introduction of HT patch clamping, as well as new assays that facilitate faster, more robust screening, there are more ion channel receptors being detected.
Researchers at the recent Society for Biomolecular Screening conference and CHI’s upcoming “Pharmacology Driven Assays for GPCRs and Ion Channels” shared information on a cornucopia of topics, including the latest enabling technologies, new screening paradigms, and novel approaches to generate GPCRs.
The IonFlux system from Fluxion Biosciences was recently beta tested by scientists at Novartis Institutes for Biomedical Research (NIBR). “Compounds, buffers, and waste are contained on a single 96-well plate, eliminating robotic handling. Air pressure drives experiments in microfluidic channels in a layer below the wells. This is a novel approach in automated electrophysiology,” explained Andrew Golden, Ph.D., post-doc fellow.
Robustness is enhanced via recordings taken from 20-cell ensembles (IonFlux HT), and pharmacology improved by recording a full range of concentrations from the same group of cells, according to the company. There are two available systems—the IonFlux 16, which uses 96-well plates, and the IonFlux HT, which uses 384-well plates.
Analysis of the prototype (alpha and beta testing) was initially focused on whether IonFlux could reproduce results demonstrated on other platforms. “The microfluidic approach could be helpful for ligand-gated ion channels—especially for subsets of those for fast desensitizing ligand-gated ion channels where you only add a short pulse of the ligand or neurotransmitter,” explained Mats Holmqvist, Ph.D., research investigator in the center for proteomic chemistry at NIBR.
In addition, Dr. Holmqvist said the hope for the new platform is that it should provide selectivity not only by target but also by function. “You can utilize ‘use dependency’—the accumulation of inhibition with repetitive depolarizations. If an ion channel is active, the drug may be much more potent.” With this new technology, one should be able to refine and understand how a compound affects an ion channel. However, it’s still too early to show whether this will be the case.
Since HT platforms for ion channels are fairly new, standardization across different instruments hasn’t been addressed. “There are different quality control parameters, including the way of recording a single cell per well or ensemble recording in parallel. Some machines use Oracle database versus file formats. We’ve been trying to address that in safety profiling. A quick answer is that we make a summary PDF file of every compound in each experiment that can be accessed any time,” noted Dr. Holmqvist.
Parallel Screening
The traditional screening paradigm involves one target for primary HTS. However, this process “wastes a considerable amount of time to get results, and also wastes efforts on compound management in order to get those compounds ready for testing,” said Peter Hodder, Ph.D., senior director of lead identification for the translation research institute at the Scripps Institute, Florida.
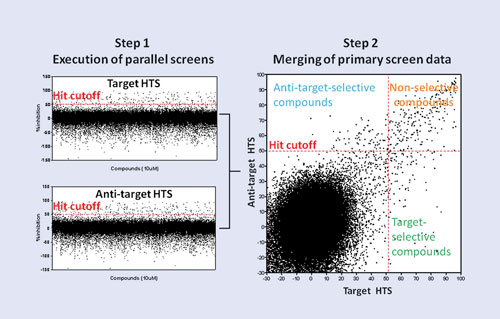
His group uses a parallel screening process that screens compounds against the target and antitarget simultaneously. “Antitarget is an all-encompassing name for any assay you would run that’s different from the target—usually to remove compounds from further consideration,” Dr. Hodder explained. “We found most of those compounds are junk compounds anyway.” The antitarget becomes important for the hit compounds, because it provides information on whether it is something specific to the target or whether it is something nonspecific to the assay format.
Time saved via parallel screening can be four to five weeks per target. In addition, and what is more important and what is harder to gauge, he noted, is saved efforts following false trails, which result in smaller, cleaner datasets. Relevant structure activity relationships emerge early in a campaign. For example, Dr. Hodder performed an SF1 (transcription factor) assay and ran the antitarget ROR against it and found potent compounds. “If we had relied on primary screening alone, those compounds would not have been selected.”
The parallel-screening format is not specific to any target class. “What’s more important is how to apply it to different target classes or different assay formats.” His group was successful in screening ion channels, including TRPML3 with TRPN1 as the antitarget (TRP is transient-receptor potential). HTS probes confirmed that the target is not located on plasma membranes in native cells.
Dr. Hodder added that this approach can be used to help focus on the most important compounds for drug or probe discovery, but it’s key is in choosing the right antitarget. “If it’s too close in relationship to the target, you’re going to start throwing out compounds you don’t want to during the campaign.”
His group is now performing more sophisticated screening using two or three antitargets and trying to find the overlap of hits that are specific in all three versus two or one of those targets and antitargets. “This challenges us to think about how we present and analyze our data.”
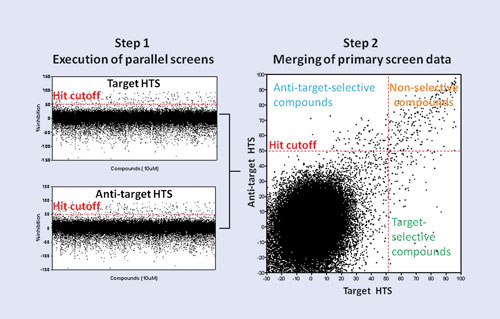
In the parallel screening process, an HTS target (e.g., GPCR, kinase) and its “antitarget” (e.g., cytotoxicity assay, selectivity assay, counterscreen) are screened as parallel HTS campaigns (left). Merging the target and antitarget primary screening data (right) facilitates rapid identification of target-selective, nonselective, and antitarget-selective compounds. [Scripps Institute, Florida]
Novel Assays
Some of the challenges of working with ion channels include controlling activity, whether with a small molecule ligand or voltage. Many ion channels inactivate within milliseconds, making HTS difficult.
David Weaver, Ph.D., director at Vanderbilt Institute of Chemical Biology HTS, has been focusing his research efforts on ion channels—especially 7TM (7-Transmembrane) receptors.
“We are interested in looking at some of the effector systems that are more physiologically relevant and one of these is the GIRK (G-protein regulated inwardly rectifying potassium (K+) channel).” His group developed this assay to measure the activity of GI-coupled 7TM receptors. “The idea was whether we could see any differences in the pharmacology and the fact that we may be using a more physiologically relevant end effector rather than using mutant G proteins to couple the change in intracellular calcium.”
The success of the GIRK assay encouraged Dr. Weaver to examine ion channels as end effectors that could be used to generate new assays with physiological relevance. Preliminary data demonstrates the ability to detect changes in M-current (muscarinic-modulated potassium current, usually studied in the brain and peripheral nervous system) activity.
He developed an HTS-compatible assay that can measure and quantify the modulation of M-current downstream from the 7TM receptor using thallium-flux. This optical assay platform can use a commercially available kinetic imaging plate reader.
According to Dr. Weaver, the only nonstandard part of the assay is that he extracts a slope from the initial measurement, instead of fitting a peak amplitude. His hope is to use this assay to further understand the pharmacology of 7TM receptors. “It’s my intent that we can demonstrate that these are good, robust assays for use in HT screens to discover novel modulators of 7TM receptors or the ion channels we’re using as effectors.”
Novel Targets
“Ion channels are terrific molecular targets, and many drugs have been targeted to them,” stated David Clapham, M.D., Ph.D., Aldo R. Castenada professor of cardiovascular research at Children’s Hospital Boston. Yet, one of the biggest challenges is the gold standard assay—the patch clamp.
This is a time-consuming technique—single cell membranes must be broken open and the current must be recorded while controlling voltage in the cell. Although HT assays exist, not all ion channels are suited to them. “The most promising are the very fast, voltage-dependent channels with large, rapid changes and ones less amenable are ones that are similar to each other in their properties, like TRP channels—these are more difficult.”
Dr. Clapham also presented information on what he thought were good, fairly recent, ion-channel targets and included some recent data on some of his work with these targets.
Many TRP channels are involved in sensory functions, like smell, taste, and hearing. TRPV3 is an ion channel that is well expressed in skin. Dr. Clapham demonstrated that both skin barrier formation and some aspects of hair formation are altered by this ion-channel’s activation or block.
It is activated by subtle temperature changes—temperatures about 32ºC—indicating TRPV3 is sensing heat at the skin surface and relating that to the nerves. This indicates it may help regulate body temperature. Growth factors such as EPGR potentiates TRPV3 to bring calcium into karatinocytes, and, in turn, TRPV3 potentiates EPGR, so there’s a positive feedback loop.
“This is important for the proper formation of skin barriers, so that there is normally a cycle of karatinocytes maturing from deeper in the skin to the surface of the skin.” Dr. Clapham added that TRP channels are difficult to work with because they are fairly slow and their properties are often difficult to distinguish. In addition, they are often small in size, and there is a lack of known ways to activate them.
Additional ion channels that Dr. Clapham thought were worth pursuing were the NAV1.7 to NAV1.9 pain targets, which are voltage-gated sodium channels. A new chloride channel, TMAM16-A, and the ORAI channel, which is important in the immune system, were also on the list. An interesting new target for contraception, called CATSPER, is an ion channel only present in mature sperm and required for male fertility. “This may be a good method of contraception without hormones,” said Dr. Clapham.
“Our job is to find new targets and new molecules, and then other people can work with those molecules to target diseases.”
New Approach
There are many challenges for the generation of new GPCRs, said Michel Bouvier, Ph.D., professor and chairman in the department of biochemistry at the University of Montreal. These include selectivity and ligand-biased signaling, where one receptor can couple to different signaling pathways in a cell.
“The problem with this is that you are trying to monitor the efficacy of a compound toward one signaling pathway, but since there are multiple ones, we don’t necessarily know which one to follow that will correlate with a disease or particular activity.” His approach is to develop one assay that could encapsulate in one reading all the signaling pathways and by dissecting the signatures, provide information about the pathways being engaged by a receptor.
Utilizing Roche’s label-free xCELLigence platform, his group is able to measure cell impedence. Each well of the plate has electrodes. As the cells grow, the impedance increases, and when the cells are treated with compounds that bind to receptors, many different pathways are triggered.
The readout reflects changes in impedance from the compound over time—providing a global assessment of the various pathways. Different compounds generate different curve shapes. “We can use this technology to differentiate classes of compounds that have different relative selectivity toward different pathways. It’s generating a simpler way to classify compounds in different efficacy profiles toward different signaling pathways.”
Dr. Bouvier added that they can now, using selective inhibitors of different pathways such as the generation of cyclic AMP, show how the inhibition influences the shape of the impedance curve. “Not only can we start classifying the ligands in different categories or compounds, but we can start making predictions on which pathways these compounds will be actively inhibiting. His group is planning to develop algorithms to apply to the curve and thus, provide a response as to which pathway is being affected. “We first need to confirm which portion of the curve informs us about each pathway.”
This approach can be used for almost any receptors, reported Dr. Bouvier. It provides a big time savings—one assay instead of four or five. However, he added, “we don’t know yet if all signaling pathways will respond to changes in impedance—from our data so far, we haven’t encountered such a pathway.”