May 15, 2010 (Vol. 30, No. 10)
Pharma Innovation Cycle Can Be Recharged Through Strategic Deployment of Technology
While the 21st century has witnessed great strides in the life science research tools industry, it has also witnessed a simultaneous decline in productivity in the pharmaceutical industry, to the extent that small molecule R&D, on average, is currently a loss-making exercise.
The problem of declining return on investment (ROI) is exacerbated by generic erosion, increased scrutiny from the FDA on the issue of drug safety, price controls and pressure from pending healthcare reform legislation, and increased hurdles for safety and/or efficacy in the new era of comparative effectiveness (CE), which essentially requires new drugs to be differentiated from those already on the market.
Even without the impact of healthcare reform or CE, prescription drug spending growth in 2008 reached its lowest level in 47 years, according to a 2009 IMS report.
At Caliper Life Sciences, we are focused on recharging the pharma innovation cycle and accelerating small molecule drug development by making each facet of the R&D process more clinically relevant. Specifically, we propose a three-pronged strategy:
- Life science tools should be judiciously used to create more efficient and productive innovation cycles through rapid, cost-effective reiterative candidate selection and optimization.
- The clinical relevance of early-stage R&D must be enhanced by making the process and data more predictive of clinical outcome.
- Early cumulative data should be leveraged to reiterate the process in order to develop new drug candidates with optimal safety, efficacy, and desirable attributes that will help differentiate the final products from a competitive standpoint.
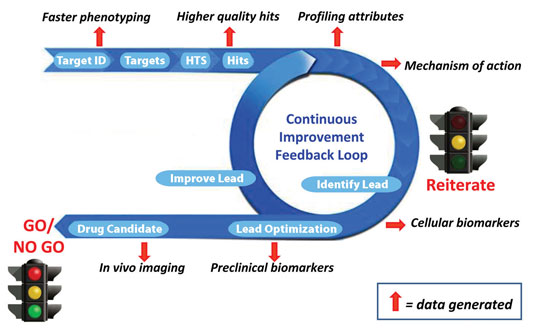
We envision implementing these complementary facets of early-stage drug development through a “continuous improvement feedback loop” (Figure). By allowing the developer to reiterate where appropriate, and to reach a go/no-go decision more quickly and with a higher level of confidence, this will ultimately mean more efficient and cost-effective drug development.
To guarantee swift and efficient execution of the innovation cycle, we contend that it is important to maintain control of these critical activities in-house, contrary to the current trend of outsourcing, which often loses sight of the importance of maintaining control of strategically valuable activities.
Despite the ability of drug developers to rapidly screen millions of compounds, such speed is moot unless the data gives an accurate assessment of key attributes, such as kinetics (on/off rates), mechanism of action, specific effects on key target enzymes, and other pharmacologically relevant data. Through strategic use of high-throughput in vitro profiling technologies, such as microfluidics-based systems, one can rapidly generate a drug profile in a cost-effective manner, allowing numerous reiterations until one or more optimal-profile candidates has been selected that has a greater chance of surviving the in vivo testing stage and beyond.
Microfluidics-based platforms enable one to rapidly screen a compound’s effect on broad classes of key enzymes, resulting in immediate feedback on the biological effects of newly synthesized compounds and can prompt another chemistry cycle in the same day rather than wait for a week, as is typically the case using existing technologies or if profiling is outsourced to a third party.
These platforms illustrate a key point that should be considered by pharma when designing early R&D strategies. Every new technology that is introduced into the innovation cycle should ideally be:
- fast (keeping pace with the preliminary screening process),
- robust (yielding reproducible data across a broad spectrum of compounds),
- cost-effective (in terms of materials and hands-on time), and
- versatile (generating multiple data points per compound).
By judiciously using new, enabling life science tools in a reiterative manner, one can quickly eliminate candidates likely to score poorly in terms of safety and efficacy, and select for additional criteria such as slower off rates, which are more likely to correlate with greater therapeutic windows once in the animal.
Given that one of the greatest drains on pharma ROI is the cost of clinical failure, it is imperative to ensure that in vitro and preclinical in vivo data is predictive of the clinical outcome. An abundance of recent technological developments, such as next-generation sequencing, genomic/proteomic biomarker discovery, companion diagnostics, and in vivo imaging with new modalities have strengthened the bridge between in vitro and in vivo research efforts, thereby facilitating more clinically relevant drug development.
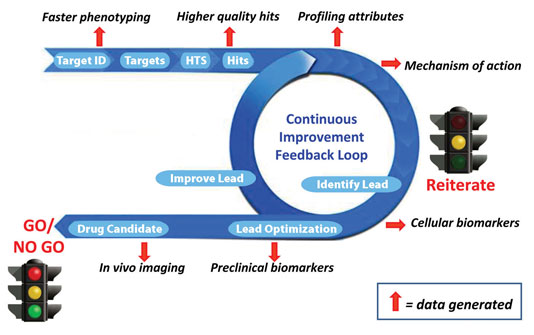
Innovation cycle for small molecule drug development
Optimizing Drug Candidate Attributes
To increase the chance of commercial success (in terms of market share, reimbursement, and pricing), a good drug candidate must be sufficiently differentiated from existing and upcoming marketed drugs with which it will be competing.
Pharmaceutical companies can introduce an early commercial success hurdle into the R&D process by using the innovation cycle reiteratively to seek a compound that has an “aspirational” profile. While the ideal profile may or may not be attainable, the drug developer can compare it with a “baseline profile” (that is, the minimal attributes for a commercial product), and make the decision to continue reiterative screening, or to proceed with the product in hand.
With an accurate and detailed knowledge of the drug candidate’s attributes at an early stage, a drug maker can quickly predict its market value, and proceed along the path that will maximize the chance of creating a safe, effective, and well-differentiated drug in order to be able to command premium pricing.
While there is no single facile solution to the pressures weighing on the pharmaceutical industry, this is a timely opportunity for reexamining the drug development process itself. The industry needs to reexamine the way in which it adopts and deploys new life science technologies.
The good news is that the current cost of enabling tools, both in terms of capital equipment and labor, has reached an attractive price point that renders this a compelling value proposition. Given the long timeline and high cost of drug development compared with the relatively modest cost of these technologies, the benefit of being able to make swift decisions at critical junctures is a simple yet tangible way of improving the ROI.
Kevin Hrusovsky ([email protected]) is CEO of Caliper Life Sciences.