October 15, 2013 (Vol. 33, No. 18)
Multiplex High-Content Imaging for Studying Compound Performance
Increasing output, reducing time, and improving accuracy of laboratory assays can be achieved by various new technologies that encompass multiplexing, low volumes, high-throughput screening, and advanced software for collection and analysis of large datasets.
Multiplexing provides the ability to obtain multiple datasets from a single assay condition.
The key benefit of multiplexing is that you gain a better and more accurate understanding of a complex system by measuring the sum of the multiple parts. If only a single activity is measured, the effect of experimental treatment on cell outcome could be overlooked. Multiplexing can also include the measurement of multiple cellular properties from a single marker, for example some nucleic acid dyes can provide information about more than one aspect of cell health. Ratios of parameters can also be calculated enabling normalization of data, for example, the ratio of a cell’s activity to the cell number in that well.
In setting up multiplexing assays, accurate optimization is required to reduce overlap between multiple signals and provide compatibility under similar conditions. There is also the requirement for equipment that can detect and analyze multiple signals in a time-dependent way. As the development of these technologies advances and more detection products become available, increasingly less time is required to optimize assays, creating a system that is more accessible and suitable for a larger number of applications.
Various detection systems are available that work on a planar or suspension system depending on the specific target required. Established chemiluminescent detection technologies are readily being replaced by fluorescent-based systems due to the advancement of a greater number of fluorophores that can be used simultaneously.
In combination with more sophisticated plate readers capable of imaging whole cells in high-throughput mode, many industries have been revolutionized, particularly drug discovery.
In this article, we discuss the identification of several hepatotoxicity effects of three commercially available compounds on a liver cell line using an advanced multiplex assay.
Unpredicted side-effects and toxicity issues limit or prevent a candidate molecule being taken to market. This can often occur very late in the testing phases, thereby incurring substantial time and cost factors for the companies involved.
Assessing cell membrane integrity is one of the most common ways to measure cell viability and cytotoxic effects. Measurements of dyes that enter only permeated cells or substances that travel outside the cell after the membrane is compromised can be used to detect these effects. In many systems, a single chemical property or kinetic response is measured to predict cytotoxicty, for example, the reducing potential of cells using a colorimetric or ATP system. These assays are based on a singleplex system where one molecule or activity is being measured. They are of low throughput and will not capture the complexity involved in cellular toxicity.
High-content screening has overcome the limitations of these existing methods by measuring several parameters simultaneously, reducing the risk of a toxic effect being missed.
Although initial studies have shown the possibility of using a high content multiplex screening program earlier in the drug development process, there are still several limitations. The software can be complex and difficult to use, requiring a lot of training, and often creating large files for storage that can be viewed only by specific software. In addition, assessment of whole wells usually requires the stitching of images together, possibly affecting data quality.
The use of TTP Labtech’s acumen® hci imager overcomes many of these challenges with increased throughput and the screening of whole wells in 384-well plates in as little as eight minutes per plate including data acquisition and analysis.
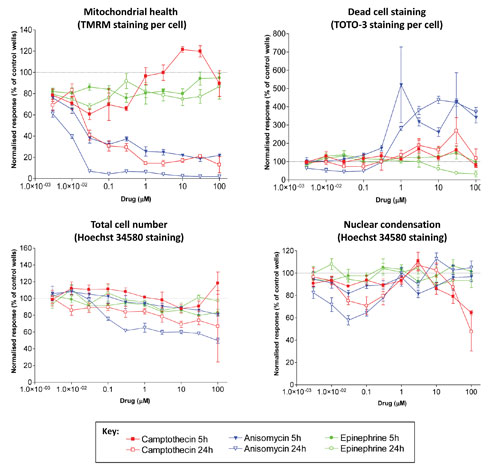
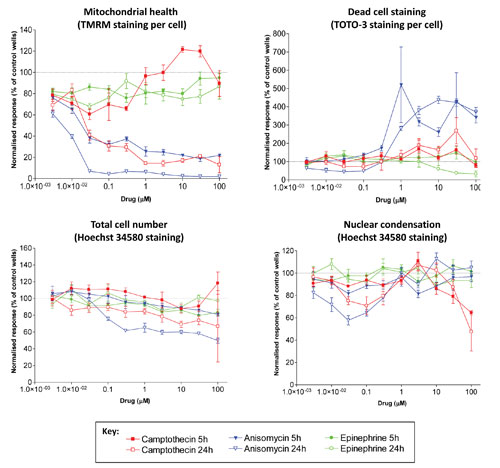
Figure 1. Concentration-dependent changes in cell health in HepG2 cells: Dotted line indicates no change relative to control wells. Data are from quadruplicate wells. Error bars indicate SEM.
In this example, we describe how the acumen hci’s three-laser scanning technology enabled four parameters of cell health to be analyzed and compared between compound-treated and vehicle control-treated HepG2 (human hepatocellular carcinoma) cells.
The 405 nm laser-excited Hoechst 34580 dye (Life Technologies™) stains all nuclei and facilitates enumeration of total cell number (giving a measure of cellular proliferation). Additionally, Hoechst nuclear half-width measurements give a measure of the nuclear condensation that occurs during apoptosis. The 488 nm laser-excited TMRM (Life Technologies) is a cell-permeant, cationic, red-orange fluorescent dye that is readily sequestered by active mitochondria. TOTO-3™ (Life Technologies) is a 633 nm laser-excitable fluorophore that stains the nucleus of dead/dying cells where the cell membrane has been compromised, thus providing a number of total dead cells per well.
Two drugs (camptothecin and anisomycin) showed concentration-dependent decreases in mitochondrial health and increases in dead-cell number that was more clearly defined after 24 hours of treatment. The third drug (epinephrine) showed minimal toxic effects at any concentration for any measure of cell health (Figure 1).
Increasing the incubation of the compounds to 72 hours enhanced the toxicity effects observed at the earlier time points. Figure 2 illustrates the significant inhibition of cell proliferation after longer term exposure to camptothecin. Of the cells that are present, a higher proportion were dead or dying (TOTO-3 staining) and had greatly decreased mitochondrial health (TMRM staining).
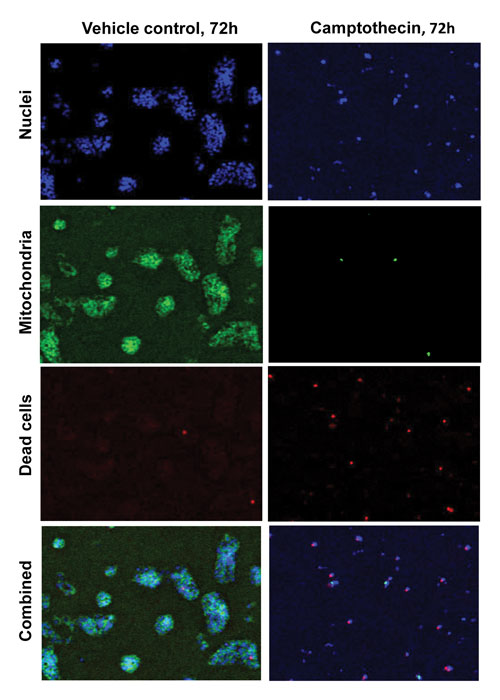
Figure 2. Treatment of HepG2 cells with camptothecin (2 µM) for 72 hours signif-icantly reduces cell proliferation, decreases mitochondrial health, and increases the proportion of dead cells. Open-source TIFF files generated by the acumen hci were false-colored using ImageJ.
Understanding the time course of the toxic effects is important for the development of a drug. Figure 3 presents a cytotoxic profile of a range of drugs over time.
In one case, etoposide showed minimal toxic effects as measured by active mitochondria, number of dead cells, and nuclear condensation, but it caused significant inhibition of cell proliferation. For each drug, different profiles may emerge, depending on the number of parameters that are measured. This is an important observation in determining the overall toxicity of a drug. If a single parameter is measured, other toxic effects could be missed, and in some cases this effect could have more serious consequences at a later stage in the drug development process.
This study highlights the need for a rapid, high-throughput, multiplex assay to analyze as many different parameters of toxicity in one assay as possible. All these functions can be carried out successfully using the acumen hci. As the development of new fluorophore reagents expands, there will be more scope for further targets to be studied within the multiplex assays.
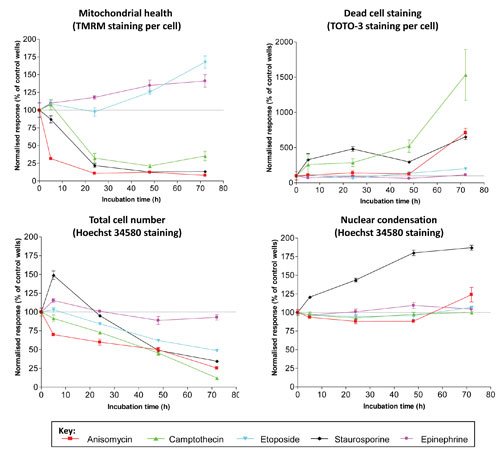
Figure 3. Time course of toxicity measurements in HepG2 cells upon treatment with 2 µM of compound. Dotted line indicates no change relative to control wells. Data are from quadruplicate wells. Error bars indicate SEM.
Sarah Burl, Ph.D. ([email protected]), is scientific communications officer at TTP Labtech.