February 1, 2014 (Vol. 34, No. 3)
From our February 1 issue.
While nurture (process optimization, media, and feed strategies) ultimately trumps nature (cell line) for maximizing protein titer, the importance of starting out with the right cell line cannot be overstated. Volumetric productivity, product quality, and the ability to meet timelines are three factors that bioprocessors balance at different stages of a product’s lifecycle.
During early cell-line optimization, time-to-clinic usually becomes the preeminent objective. Alison Porter, head of mammalian cell culture R&D at Fujifilm Diosynth Biotechnologies, a contract research and manufacturing firm, notes that productivity and quality attributes must eventually reach the point of commercially viability, “but ultimately it comes down to speed to reach first-in-human studies. Of course you want good titers, but you don’t have to put in all that extra work at such an early stage to get the highest possible titer. A merely good titer is satisfactory as long as clinical timelines are met.”
While much can be said for controlling every aspect of cell-line development, sponsors generally trust their contractors to select the specific cell line, screening, and processes that will achieve the ultimate goal of timeliness. They expect well-characterized cell lines and transfection platforms with which regulators have ample experience. “In the end they’re looking for a sensible process,” Porter says.
One of the most significant trends in cell-line development, according to Porter, is wide-scale adoption of microbioreactor and shaken microplate systems capable of fed-batch operation. “These technologies have allowed investigators to look into larger numbers of cell lines in systems that are representative of the bioreactor process. They allow better decision-making on which cell lines to progress because they transfer well to process development, where they are used in design-of-experiment to optimize cells and process conditions.”
Before the advent of the microbioreactor, static screens and shake flasks dominated cell line and process development. But the flasks are relatively bulky and difficult to control for common process parameters. Now, shaken microplates replace static screens and microbioreactors take the place of flasks. “Microbioreactors are a step closer to bioreactors,” Porter says.
Ready-Made Cells
Mark Melville, Ph.D., senior director of bioprocess development at EPIRUS Biopharmaceuticals, notes that two main avenues to cell-line development are open to virtual companies like EPIRUS.
The more traditional approach involves working with a company that builds cell lines from the ground up using a suitable expression system and host cell line, followed by conventional screening. For biosimilars, EPIRUS’ specialty, quality endpoints would be brought into the development process as early as feasible.
The second strategy, which has become more common since the advent of biosimilars, is to in-license cell lines optimized for the expression of specific biosimilars, for example trastuzumab (Herceptin) or bevacizumab (Avastin).
In-licensing cells significantly has the potential to shorten development times. “But there are tradeoffs,” Dr. Melville cautions. “If you’re going that way you don’t have a wide range of cell lines to select from. By comparison, when working from scratch the sponsor can influence and shape development all along the way. In-licensing may save time, but it adds risk.”
The risk is that, in a new set of hands, the cell line may not meet productivity or quality goals. “These vendors will provide cells, but they cannot guarantee success—that you’ll have an approvable biosimilars if you use their product,” Dr. Melville says. “There are many steps between receiving cells and achieving a commercial process.”
An interesting analogy might be purchasing a component or ingredient for a gourmet dinner rather than making it from scratch. An inexperienced cook probably has a higher expectation of success going this route, but professional chefs know the result will be perfect if they control every “unit operation” in preparing that meal. In other words, companies inexperienced with cell-line development are probably better off in-licensing.
For the in-licensing scenario the risk of not reaching specified quality attributes is a lot more serious than for not achieving viable productivity. “It’s not difficult to achieve commercially viable titers,” Dr. Melville explains. Quality, particularly for biosimilars developers, involves hitting a narrow target of physico-chemical similarity with the innovator drug. Factors such as culture method and medium, feed strategies, and purification all affect quality. Companies that in-license must still perform significant development on these components of a successful process. Furthermore, the analytical methods that originally qualified product-specific cells may be inadequate for demonstrating biosimilarity to regulators.
Dr. Melville is not at liberty to divulge his company’s strategy with respect to sourcing cell-line development services. He did say, however, that most established biomanufacturers tend to keep as much control as possible over cell-line development, except for “selective” cases that make good business sense.
Fast, Stable Transfection
Transient transfection provides a rapid route to modest quantities of protein for characterization and preclinical investigation. Now, a group at the National Research Council of Canada has devised a rapid, stable transfection method which, although somewhat slower than transient transfection, provides several advantages under the right circumstances.
Yves Durocher, Ph.D., team leader for protein production at the NRC, uses a promoter developed at the NRC and a recently acquired CHO cell line. Dr. Durocher and co-workers have adapted the line to serum-free suspension, generated a master cell bank, and validated the line for use in biomanufacturing.
Within this CHO cell line, CHOBRI/Cum2, researchers combined elements (a transactivator and repressor) that allow for cumate-regulated expression. This platform generates CHO pools that natively, stably express between 200 and 500 mg/L of protein in less than four weeks post-transfection: two weeks for clone selection, and two for production. “We have shown that the pools can be maintained for months in culture under selection pressure,” says Dr. Durocher, “and maintain productivity at 200 to 500 mg/L.”
Application of simple feed strategies push productivity to 800 mg/L, with certain clones isolated from the pools showing productivity up to about 1.5 g/L. “Our goal is to double that titer within the next six months,” Dr. Durocher notes.
This expression platform works for membrane proteins as well as monoclonal antibodies. The NRC group has developed cell lines expressing GPCRs and other transmembrane proteins. According to Dr. Durocher, flow cytometry analysis shows that for selected pools, as many as 99% of the cells are positive for the surface proteins.
Because it takes approximately twice as long as transient transfection, this platform is impractical for high-throughput, early-stage candidate selection, but it excels when protein candidates have been reduced to about 10 or fewer. Here, the extra two weeks to protein product carries a substantial reward: a manufacturing-worthy, stably transfected cell line.
“Using stably transfected cell pools to generate research material reduces differences you’d normally see by changing the host or production method,” Dr. Durocher explains. “While it does not replace transient transfection, it offers an interesting platform alternative.” Another advantage is greater consistency in quality attributes between early research material and preclinical batches.
This technology is available for licensing from the NRC. One company is already using it, and discussions are ongoing with several others.
More than Transcription
Creation of cell lines expressing recombinant protein therapeutics begins with transfection, often in CHO cells. Though the introduction of foreign DNA into mammalian cells has become routine, stable gene integration occurs infrequently. Thus, the generation and isolation of stable transfectants from the large pool of untransfected, or transiently transfected cells can be laborious and time-consuming.
The SUREtech platform from Selexis is based on the use of unique Selexis Genetic Elements (SGEs) inserted within the expression vectors harboring the gene of interest. The SGEs control the dynamic organization of chromatin. “These genetic elements have been identified in silico using a bioinformatic tool, and are ubiquitous sequences interspersed in the genome of all eukaryotic cells,” explains Pierre-Alain Girod, Ph.D., chief scientist.
SGEs work by organizing chromatin in topological loops. Once integrated into an expression vector, these elements enable greater efficiency of stable transfection because they create a topological loop akin to supercoiled DNA. “The result is higher numbers of stable clones,” Dr. Girod explains.
In addition, SGEs act at the transcription level for the gene of interest, to enable higher expression. They create what Dr. Girod calls “a nucleosome-free gap” that enables transcription factors to access regulatory sequences on the gene. This function is further strengthened as the SGEs act as a “sink” for RNA polymerase II. Thus, they stabilize expression by keeping the cassette of the gene of interest open, which prevents silencing.
But elevated transcription alone does not guarantee optimal productivity. This is due to CHO cells’ limited phenotypic characteristics. A new approach engineers host cells to overcome the diminution of the cell’s “fitness.”
In a recent paper, scientists from Selexis and the University of Lausanne described engineering an entire secretion pathway in CHO cells to successfully produce a biosimilar antibody version of infliximab. “We created ‘perfect’ host cells that co-expressed multiple chaperones to complement the cell metabolic limitations,” says Dr. Girod. This approach brought about stable phenotype improvements. For example, it de-bottlenecked folding of the infliximab light chain, which facilitated secretion and production of the antibody within the CHO cell’s supernatant.
Designer Antibodies?
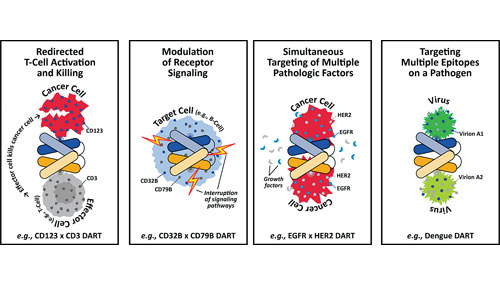
One of the more intriguing cell-line engineering feats is illustrated by Dual Affinity Re-Targeting (DART®) proteins, which are antibody-like therapeutic proteins that target multiple, different epitopes. Under direction from Valentina Ciccarone, Ph.D., principal scientist, MacroGenics, investigators have expressed DART in CHO cells using vector systems similar to those used for expression of conventional antibodies.
“Protein expression levels vary depending on the specific molecule and structure, and one or more vectors may be used to express the multiple peptide chains,” Dr. Ciccarone explains. “We have not observed toxicity to the host cells as a result of DART protein expression.”
The multiple affinity regions on DART molecules are linked covalently. Due to the lack of an Fc region, basic DARTs have a shorter half-life than conventional antibodies. MacroGenics has achieved half-life extensions by further engineering the molecules with an Fc region or albumin binding domain (ABD), or through PEGylation.
Where cell-line work for conventional antibodies is often concerned with expression levels, for DART the overriding issue is product quality. DART assembly within the host cells requires optimal expression levels and correct assembly of two, three, and sometimes four peptide chains.
“Our approach for DART cell-line development involves screening candidate cell lines for titer as well as for product quality,” Dr. Ciccarone continues. “This has been a successful approach, and to date we have been able to manufacture several DART molecules with varying target affinities and structures. In each case have achieved expression levels greater than 1 g/L with acceptable product quality.”
MacroGenics has engineered more than 100 DART molecules in mammalian cells. These molecules bind, with relevant biological activity, to multiple targets: CD123 × CD3; CD19 × CD3; CD32B × CD79B; EGF-R × IGF-1R; as well as multiple epitopes of smallpox and dengue viruses.
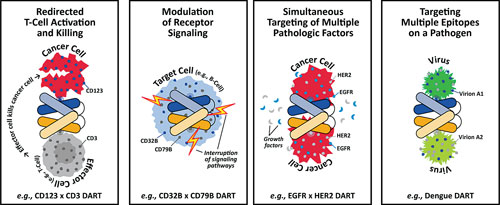
MacroGenics’ DART® proteins target multiple, different epitopes.