December 1, 2009 (Vol. 29, No. 21)
Alterations in the Expression of miRNA Genes Contribute to Pathogenesis on Broad Basis
MicroRNA (miRNA) has a significant role in controlling developmental and cancer processes like cell proliferation, differentiation, cell cycle, apoptosis, and metastasis. This ubiquitousness and their recently revealed role as key regulators of gene expression during development has boosted their use as agents in the fight against cancer. It’s a hot topic “and it’s getting hotter,” said Frank Slack, associate professor of molecular, cellular, and developmental biology at Yale University, at the Keystone Symposium “MicroRNA and Cancer” held earlier this year.
According to Curtis Harris, M.D., chief, laboratory of human carcinogenesis at the NIH, “throughout the community, there is a lot of interest in translational science, which you can see from the number of papers published in the last few years.”
It’s generally accepted that alterations in the expression of miRNA genes contribute to the pathogenesis of most, possibly all, human malignancies. “We found that these alterations can be caused by various mechanisms, including deletions, amplifications, or mutations involving miRNA loci, epigenetic silencing, or the dysregulation of transcription factors that target specific miRNAs,” noted Carlo Croce, M.D., director of human cancer genetics at the Ohio State University Comprehensive Cancer Center.
“Because malignant cells show dependence on the dysregulated expression of miRNA genes, which in turn control or are controlled by the dysregulation of multiple protein-coding oncogenes or tumor suppressor genes, these small RNAs provide important opportunities for the development of future miRNA-based therapies.”
At the meeting, Dr. Croce presented some of his group’s discoveries on the role that miR-21 plays in the EGF receptor pathway in cancer. “It’s an interesting issue; there is either loss of expression or overexpression of miRNAs in cancer. We found in particular that miR-21 is dysregulated in six out of six solid tumors.
“We have found dysregulation of miR-21 in 13 out of 13 different types of malignancies. By microarray and RT-PCR, we now know that miRNAs are dysregulated by pathways that are involved in cancer. The fact that miRNAs are downstream targets of pathways involving cancer provides tremendous opportunity for therapy. Many components of the pathway causing cancer may be undruggable. On the contrary, miRNAs that are downstream targets are druggable.”
Dr. Croce showed that the global expression of miRNA indicated specific signatures for every human cancer. “We did a systematic study and discovered how each miRNA is dysregulated, and discovered signatures for early and late stages of human cancer. In the future, we’ll use this information to develop miRNA therapies.”
Inflammation
Dr. Harris’ group is focused on the molecular epidemiology of human cancer and clinical biomarkers of cancer diagnosis, prognosis, and therapeutic outcomes, and miR-21 is of particular interest. “miR-21 is associated with both lung cancer and the epidermal growth factor (EGF) pathway,” said Dr. Harris. He noted that a particular area of clinical interest is EGF’s binding receptor EGFR and its role in lung cancer in women who are nonsmokers.
“miR-21 is increased in lung cancer and at least 13 types of cancer. As initially discovered by Dr. Croce and George Calin at Ohio State University, miR-21 is over-expressed in many cancers. So from a predictive prognosis standpoint and promising preclinical studies, miR-21 is a key therapeutic target for future clinical studies.
“There are many links between inflammation and cancer, and studies have shown that inflammation predisposes the development toward cancer,” he added. “We are especially interested in early-stage colon cancer. Some of our work, which was previously published in JAMA, looked at changes in miR-21 and found that those changes were predictive of prognosis and therapeutic outcome. We decided to put both miRNA and inflammatory gene biomarkers together and asked the question, ‘Do you get a better prognostic indicator?’ And the answer is yes, you do.”
Dr. Harris’ group is also investigating the interaction between nitric oxide (NO) and p53 as a crucial pathway in inflammatory-mediated carcinogenesis and has shown that NO induces ATM- and ATR-dependent p53 post-translational modifications leading to a p53 stress response in human cells in vitro, which can modulate the risk of cancer in colon tissue from patients with ulcerative colitis or Crohn’s, both of which are cancer-prone, chronic inflammatory diseases.
In the future, miRNA studies need to “investigate a larger number of patients, comparing cancer biomarkers across ethnic groups so there’s a large body of data to analyze,” noted Dr. Harris. “It’s also time to do prospective studies; most of the reported studies have been retrospective. In the field of miRNAs, more and more people are getting involved every day. And the results are translating rapidly into the clinic. There is a lot of commercial interest in this area.”
Radiation
Dr. Slack has a novel approach to miRNA research. “My lab comes into miRNA studies with the perspective of ‘what’s in it for the animal?’. Papers have been published on miRNAs based on cell and tissue culture, but we have done much of our miRNA work in vivo in C. elegans and mouse.”
Dr. Slack talked about his lab’s work on miR-34 and its response to radiation exposure. “miR-34 was first discovered in C. elegans, and a bunch of labs discovered that miR-34 was directly downstream of p53. Some groups showed that P53 was part of miR-34. We looked at the proliferation of miR-34 in p53’s role in cancer and its response to radiation.”
When Dr. Slack first started working on miR-34, no one had yet knocked out miR-34. His group found that C. elegans with loss-of-function mutations in the miR-34 gene have an abnormal cellular survival response to radiation. “These animals are highly radiosensitive in the soma and radioresistant in the germline, showing a role for miR-34 in both apoptotic and nonapoptotic cell death in vivo, much like that of cep-1, the C. elegans p53 homolog,” he added.
His group’s findings were additionally validated in vitro in breast cancer cells, where exogenous addition of miR-34 alters cell survival post-radiation. “So what we found confirms that miR-34 is required for a normal cellular response to DNA damage in vivo, resulting in altered cellular survival post-irradiation,” said Dr. Slack. “And this indicates potential therapeutic use for anti-miR-34 as a radiosensitizing agent in p53-mutant breast cancer.”
Applied Therapeutics
Josh Mendell, associate professor of genetic medicine at the Johns Hopkins University School of Medicine, said that his lab has had a long-standing interest in miRNAs.
“We have been particularly interested in understanding how miRNAs are regulated in cancer cells and how aberrantly expressed miRNAs influence cancer cell behavior. We have found that specific miRNAs are often downregulated in cancer cells and restoration of expression of these miRNAs can potently inhibit tumorigenesis. This led us to test the idea that delivery of such antitumorigenic miRNAs could represent a novel therapeutic strategy for cancer.”
Dr. Mendell’s team focuses on liver cancer for many reasons. “The liver is one of the most accessible tissues for delivery of nucleic acid-based therapeutics. So it made sense to try this strategy first in a tissue where delivery would present less of a technical obstacle. Additionally, hepatocellular carcinoma is an aggressive cancer for which few, if any, effective therapies exist. So there is a great clinical need for new therapeutics for this disease.
“To deliver the microRNA in our study, we teamed up with Reed Clark and Jerry Mendell’s groups at Nationwide Children’s Hospital to develop adeno-associated virus (AAV) vectors that would express the miRNA. This is a nontoxic viral gene delivery platform, currently being used in clinical trials for other diseases that can efficiently carry gene products to the liver.”
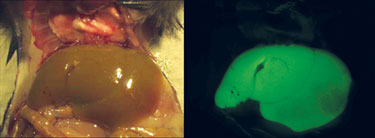
Dr. Mendell’s team demonstrated that hepatocellular carcinoma (HCC) cells exhibit reduced expression of miR-26a, a miRNA that is normally expressed at high levels in diverse tissues. They showed that expression of this miRNA in liver cancer cells in vitro induces cell-cycle arrest associated with direct targeting of cyclins D2 and E2.
“Systemic administration of this miRNA in a mouse model of HCC using AAV results in inhibition of cancer cell proliferation, induction of tumor-specific apoptosis, and dramatic protection from disease progression without toxicity. We were excited by the potent therapeutic response as well as the apparent lack of toxicity of the miRNA treatment strategy. Since miR-26a is also lost in human HCC tumors, we think that with further study, this strategy may eventually see clinical use.”

Researchers at Johns Hopkins have shown that miR-26a in a mouse model of hepatocellular carcinoma inhibits cancer cell proliferation. (Janaiah Kota, Nationwide Children’s Hospital)
Leukemia
Florian Kuchenbauer, M.D., of the medical department at the University of Ulm, is an authority on the role of miRNA in leukemia. While the functional role of miRNA is becoming well defined, the role of passenger miRNA, or miRNA*, is still not clear. “We led the concept further through next-generation sequencing, allowing us to group miRNA/miRNA* duplexes. Furthermore, we tried to show involvement of both miRNA duplex strands in leukemia and normal hematopoiesis. Taken together, the novelty of the concept is that both miRNA strands can be potentially active when expressed and that miRNA and miRNA* strands underlie a dynamic regulation.”
Dr. Kuchenbauer investigated nine deep-sequencing libraries from a variety of human and murine cells and used these to determine the most abundant complementary strand for nonannotated miRNA*. He then calculated the ratio of miRNA/miRNA* for each miRNA duplex. In contrast to previous assumptions that one strand is highly dominant, “we found that only approximately 50% of the investigated miRNA duplexes exhibit high ratios with a dominating strand, 20% have intermediate ratios, and a remarkable 10% show low ratios, which indicate comparable expression of both strands.”
Comparing miRNA/miRNA* ratios across the miRNA sequence libraries revealed that most ratios remain constant across tissues and species.
“This could possibly allow for a novel classification of miRNAs into α-duplexes, miRNAs duplexes with a dominant strand, and α-duplexes with both strands being abundant,” concluded Dr. Kuchenbauer.