City of Hope scientists have started a first-in-man clinical trial to evaluate a chimeric antigen receptor (CAR) T cell therapy for glioblastoma (GBM) that uses a scorpion venom peptide called chlorotoxin (CLTX) to direct T cells to target brain tumor cells. The researchers have just published details of preclinical studies with the technology, which showed that treating mouse models of GBM using the CLTX-CAR T cells resulted in tumor regression, without evidence of off-target effects.
“Our chlorotoxin-incorporating CAR expands the populations of solid tumors potentially targeted by CAR T cell therapy, which is particularly needed for patients with cancers that are difficult to treat such as glioblastoma,” said Christine Brown, PhD, City of Hope’s Heritage Provider Network professor in immunotherapy and deputy director of T Cell Therapeutics Research Laboratory. “This is a completely new targeting strategy for CAR T therapy with CARs incorporating a recognition structure different from other CARs.”
Brown and colleagues reported on their preclinical studies in Science Translational Medicine, in a paper titled, “Chlorotoxin-directed CAR T cells for specific and effective targeting of glioblastoma.”
Glioblastoma is the most common form of primary brain tumor (PBT) but is also among the most deadly. It is particularly difficult to treat because the tumors are disseminated throughout the brain, and efforts to develop immunotherapies, including CAR T cells, for GBM have to contend with a high degree of heterogeneity within the tumors. “Despite increasingly aggressive treatments incorporating surgery, chemotherapy, and radiotherapy, survival of patients with GBM has only modestly improved over the past several decades,” the authors wrote. More recent therapies, including GBM-targeting CAR T cells, have also provided overall response rates that have been “unsatisfyingly low,” the team noted, and this is at least in part because of this high tumor heterogeneity.
Chimeric antigen receptors design commonly incorporates a monoclonal antibody sequence in the targeting domain, which enables the CAR T cells to recognize antigens and kill tumor cells. In contrast, the approach taken by the City of Hope team exploited the tumor-binding propensity of the 36-amino acid CLTX peptide, which was originally isolated from death stalker scorpion (Leiurus quinquestriatus). “CLTX has been established to bind broadly and specifically to GBM and other neuroectodermal tumors while showing minimal cross-reactivity with non-malignant cells in the brain and elsewhere,” the scientists noted.
The investigators engineered the CLTX peptide to act as the CAR recognition domain of a CAR T cell immunotherapy technology, and tested binding in human GBM patient-derived cell lines with the expression of antigens currently under investigation as CAR T cell targets, including IL13Rα2, HER2, and EGFR. They found that CLTX bound to a greater proportion of patient tumors, and cells within these tumors. CLTX binding was also demonstrated against the GBM stem-like cells that are thought to seed tumor recurrence. “… these studies confirm the broad GBM-binding potential of CLTX, providing rationale for investigating its use for CAR T cell immunotherapy,” they wrote.
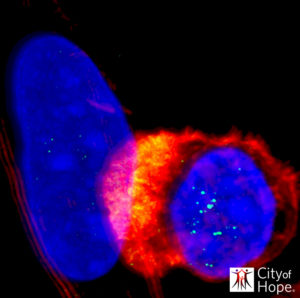
Encouragingly, further experiments confirmed that the CLTX-CAR T cells recognized and killed broad populations of GBM cells, but not non-tumor cells in the brain and other organs. The scientists’ studies demonstrated that CLTX-directed CAR T cells were highly effective at selectively killing human GBM cells in cell-based assays and in animal models, without off-tumor targeting and toxicity. “The antitumor effects of CLTX-CAR T cells were robust and specific,” they noted. It had previously been reported that CLTX binding is associated with multiple membrane proteins. The City of Hope team’s investigations further suggested that CLTX-CAR T cell activation was dependent on membrane-associated, but not soluble, matrix metalloproteinase 2 (MMP).
“Our study thus establishes that the intrinsic binding properties of a natural toxin peptide can be exploited in generating targeted CAR T cells,” the authors concluded. “This study demonstrates that CLTX, a peptide component of scorpion venom, can be successfully incorporated into a CAR construct to redirect cytotoxic T cells to target GBMs.” And, as the team further noted, although the mouse models may only represent a surrogate for human safety, they still provide relevant information about off-tumor targeting. “Using these preclinical models, we determined that systemic (intravenous) and regional (ICT and ICV) delivery of CLTX-CAR T cells into both healthy and tumor-bearing mice did not show systemic toxicity.” The investigators say their combined results provide “strong evidence” for efficacy, together with negligible off tumor toxicity, and so “support future clinical evaluation in patients with GBM.”
Michael Barish, PhD, City of Hope professor and chair of the department of developmental and stem cell biology, initiated the development of a CAR using chlorotoxin to target GBM cells. The peptide has been used as an imaging agent to guide GBM resection surgery, and to carry radioisotopes and other therapeutics to GBM tumors.
“Much like a scorpion uses toxin components of its venom to target and kill its prey, we’re using chlorotoxin to direct the T cells to target the tumor cells with the added advantage that the CLTX-CAR T cells are mobile and actively surveilling the brain looking for appropriate targets,” Barish said. “We are not actually injecting a toxin, but exploiting CLTX’s binding properties in the design of the CAR. The idea was to develop a CAR that would target T cells to a wider variety of GBM tumor cells than the other antibody-based CARs. The notion is that the higher the proportion of tumor cells that one can kill at the beginning of treatment, the greater the probability of slowing down or stopping GBM growth and recurrence.”
Dongrui Wang, a doctoral candidate in City of Hope’s Irell & Manella Graduate School of Biological Sciences, was the lead scientist to establish and optimize the CLTX-CAR T cell platform and to determine that cell surface protein matrix metalloprotease 2 is required for CLTX-CAR T cell activation. Wang added, “While people might think the chlorotoxin is what kills the GBM cells, what actually eradicates them is the tumor-specific binding and activation of the CAR T cells.”


