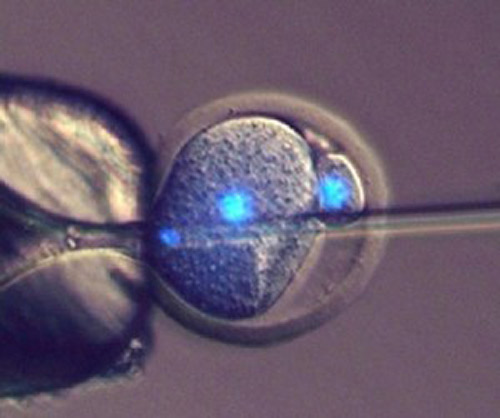
![Scientists at Bath have developed a method of injecting sperm into a one-cell mouse embryo, which can go on to produce offspring. [University of Bath]](https://genengnews.com/wp-content/uploads/2018/08/Sep14_2016_InjectingMiceEmbryo1116337202-1.jpg)
Scientists at Bath have developed a method of injecting sperm into a one-cell mouse embryo, which can go on to produce offspring. [University of Bath]
Researchers from the University of Bath in the U.K. say they have shown for the first time that offspring can be made from non-egg cells. They point out that their discovery that challenges two centuries of received wisdom
Eggs can be “tricked” into developing into an embryo without fertilization, but the resulting parthenogenotes die after a few days because key developmental processes requiring input from sperm don’t happen. However, scientists from the department of biology and biochemistry at the University of Bath have developed a method of injecting mouse parthenogenotes with sperm that allows them to become healthy baby mice with a success rate of up to 24%. This compares to a rate of 0% for parthenogenotes or about 2% for nuclear transfer cloning.
The study (“Mice Produced by Mitotic Reprogramming of Sperm Injected into Haploid Parthenogenotes”) is published in Nature Communications.
“This is first time that full-term development has been achieved by injecting sperm into embryos [during a particular point in the mitotic cell cycle],” said Tony Perry, Ph.D., molecular embryologist and senior author of the paper. “It had been thought that only an egg cell was capable of reprogramming sperm to allow embryonic development to take place. Our work challenges the dogma, held since early embryologists first observed mammalian eggs around 1827 and observed fertilization 50 years later, that only an egg cell fertilized with a sperm cell can result in a live mammalian birth.”
The research project stemmed from an idea by Toru Suzuki, Ph.D., from Dr. Perry’s team in the laboratory of mammalian molecular embryology. The group performed the study with colleagues from the University of Regensburg and the Fraunhofer Institute for Toxicology and Experimental Medicine in Germany.
The baby mice born as a result of the technique seem completely healthy, but their DNA started out with different epigenetic marks compared with normal fertilization. This suggests that different epigenetic pathways can lead to the same developmental destination, something not previously shown.
“Remodeling of histones and genomic 5′-methylcytosine and 5′-hydroxymethylcytosine following embryo injection were distinct from remodeling in fertilization and the resulting 2-cell embryos consistently possessed abnormal transcriptomes,” wrote the investigators. “These studies demonstrate plasticity in the reprogramming of terminally differentiated sperm nuclei and suggest that different epigenetic pathways or kinetics can establish totipotency.”
The team added that their research shows that “most of the first embryonic cell cycle can be bypassed in sperm genome reprogramming for full development.”
The discovery has ethical implications for recent suggestions that human parthenogenotes could be used as a source of embryonic stem cells because they were considered inviable. It also hints that in the long-term future it could be possible to breed animals using non-egg cells and sperm. Although this is still only an idea, it could have potential future applications in human fertility treatment and for breeding endangered species.