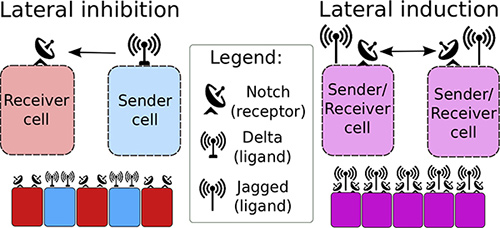
Notch signaling, the mechanism by which neighboring cells influence each other’s fates, may be hijacked by cancer cells, particularly if they adopt a previously unrecognized phenotype, a Sender/Receiver hybrid phenotype. To date, Notch signaling was deemed to rely on a ligand called Delta to set up a kind of intercellular toggle switch, one that would give rise to two distinct phenotypes, Sender (high ligand, low receptor) and Receiver (low ligand, high receptor). Yet recent work has devoted increasing attention to another ligand called Jagged. With Jagged in the mix, the Notch circuitry may behave like a three-way switch, giving rise to a hybrid phenotype.
Called Sender/Receiver (medium ligand, medium receptor), this hybrid phenotype allows neighboring cells to both send and receive signals, which can then attain similar fates, rather than the contrasting fates one might expect if Delta were all, and Jagged were inconsequential. Since this kind of two-way signaling permits the broadcasting of “be like me” messages, rather than “be not like me,” it is vulnerable to misuse by cancer cells, which are all too eager to recruit healthy cells to their nefarious cause.
According to computational analyses carried out by biophysicists at Rice University, cancer cells may essential form roving gangs of Sender/Receiver hybrids that migrate together, wantonly spreading their Sender/Receiver ways. These scientists, led by Eschel Ben-Jacob and Jose Onuchic, presented their findings January 20 in the Proceedings of the National Academy of Sciences, in an article entitled, “Jagged–Delta asymmetry in Notch signaling can give rise to a Sender/Receiver hybrid phenotype.” They emphasized that their work suggests new perspectives not only on tumor-stroma signaling, but on embryonic development and wound healing.
We find that the asymmetric effect of [the Notch Intracellular Domain gives rise to a hybrid Sender/Receiver phenotype]. This phenotype allows neighboring cells to both send and receive signals, thereby attaining similar fates,” wrote the authors. “We also show that due to the asymmetric effect of the glycosyltransferase Fringe, different outcomes are generated depending on which ligand is dominant: Delta-mediated signaling drives neighboring cells to have an opposite fate; Jagged-mediated signaling drives the cell to maintain a similar fate to that of its neighbor.”
“At the heart of our new understanding is that the primary agents of metastasis are clusters of hybrid epithelial (nonmobile) and mesenchymal (migrating) cells,” Dr. Ben-Jacob said. “These, and not the fully mesenchymal cells, are the ‘bad actors’ of cancer progression that pose the highest risk. By acting together, these hybrid cancer cells have a better chance to evade the immune system during migration and can better survive while circulating in blood vessels.”
“Cancer takes advantage of jagged proteins’ influence to form what are essentially migrating units of hybrid cancer stem cells,” Dr. Ben-Jacob added. Notch-jagged signaling also helps cells develop resistance to chemotherapy and radiotherapy and facilitates metastasis formation by promoting communications between cancer and stromal (connective tissue) cells at the new locations.
Recent findings showed stromal cells in the tumor environment secrete Jagged ligands. The Rice researchers found cancer cells hijack nearby stromal cells and prompt them to boost their production of the ligand, reinforcing the cancer's chances of survival.
The researchers suggested cells' internal expression of Jagged may also increase the production and maintenance of therapy-resistant cancer stem cells.