![A genome-wide RNAi screen was used to assess the effects of 15,000 genes on the balance between self-renewal and differentiation of human hematopoietic stem cells (HSCs). The screen identified candidate genes whose knockdown maintained the HSC phenotype during culture. Such findings could lead to better protocols to grow these cells outside the body, potentially making bone marrow transplants more available to patients suffering blood cancers, or even identifying novel genes to target during the treatment of leukemia (left and right panels). Four genes in particular implicated cohesin, a ring-like protein complex that binds to the DNA in all of our cells, in the control of self-renewal versus differentiation in HSCs. Deficiency of cohesin causes an increase in self-renewal and a decrease in differentiation of HSCs. [Cell Reports]](https://genengnews.com/wp-content/uploads/2018/08/Mar23_2016_CellReports_15000GeneEffects7512517723-1.jpg)
A genome-wide RNAi screen was used to assess the effects of 15,000 genes on the balance between self-renewal and differentiation of human hematopoietic stem cells (HSCs). The screen identified candidate genes whose knockdown maintained the HSC phenotype during culture. Such findings could lead to better protocols to grow these cells outside the body, potentially making bone marrow transplants more available to patients suffering blood cancers, or even identifying novel genes to target during the treatment of leukemia (left and right panels). Four genes in particular implicated cohesin, a ring-like protein complex that binds to the DNA in all of our cells, in the control of self-renewal versus differentiation in HSCs. Deficiency of cohesin causes an increase in self-renewal and a decrease in differentiation of HSCs. [Cell Reports]
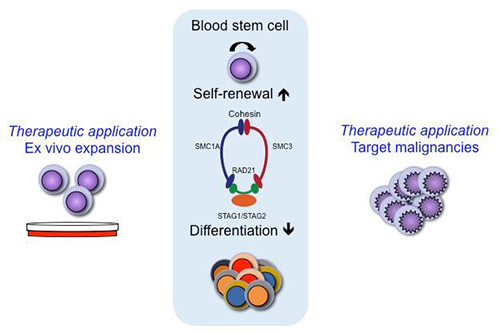
Best known for its ability to regulate the separation of sister chromatids during cell division, the cohesin protein complex, a ring-shaped structure, has shown that it has other powers, such as the facilitation of DNA repair and the modification of transcription. And now, according to scientists based at Lund University, there is evidence that the cohesin complex controls the growth of blood stem cells. More to the point, the cohesin complex determines whether blood stem cells self-renew or differentiate.
The new finding is significant because it can help scientists improve the expansion of blood stem cells outside the body, thus increasing the supply of blood stem cells to patients suffering leukemia or hereditary blood disorders. Besides making bone marrow transplant material more available, the new finding could point scientists to new points of attack for the treatment of blood cancer, which is a disruption between blood stem cell multiplication and maturation.
The Lund University scientists, led by Jonas Larsson, presented their results March 17 in the journal Cell Reports, in an article entitled “Genome-wide RNAi Screen Identifies Cohesin Genes as Modifiers of Renewal and Differentiation in Human HSCs.” The article describes how a genome-wide RNA interference (RNAi) screen was performed in primary human CD34+ cells. This screen enabled the scientists to identify candidate genes whose knockdown maintained the HSC phenotype during culture.
“A striking finding was the identification of members of the cohesin complex (STAG2, RAD21, STAG1, and SMC3) among the top 20 genes from the screen,” wrote the authors. “Upon individual validation of these cohesin genes, we found that their knockdown led to an immediate expansion of cells with an HSC phenotype in vitro.”
A similar expansion, the authors added, was observed in vivo following transplantation to immunodeficient mice.
“Transcriptome analysis of cohesin-deficient CD34+ cells showed an upregulation of HSC-specific genes,” the authors continued. This finding, the authors asserted, demonstrates that when cohesin is deficient, transcription shifts to a more stem cell–like pattern.
“The research is unique as the study of so many genes alongside one another is unprecedented,” said Dr. Larsson. “In addition, we have used human blood stem cells, which is difficult in itself as it is requires the gathering of a large amount of material.”
Of the 15,000 genes that were tested, the Lund team found around 20 candidates with a strong capacity to affect the balance of growth in the blood stem cells. What was striking was that four of these 20 genes were physically connected through cooperation in a protein complex.
“The discovery showed that this protein complex is crucial and has an overarching function in the growth of the blood stem cells,” emphasized Dr. Larsson.
The cohesin complex acts as a sort of brace that holds different parts of the DNA strand together in the cell. The researchers believe that this allows the cohesin complex to control access to the “on/off switches” in DNA and to change the impulses the blood stem cells receive from various genes, thereby affecting cell division. The blood stem cell either multiplies or matures to become a specialized cell with other tasks.
Independently of the Lund researchers' discovery, other research in the field of blood cancer has recently identified mutations in exactly the same four genes in patients with various forms of blood cancer.
“This is incredibly exciting! Together with the results from our study, this indicates that the cohesin genes are directly and crucially significant in the development of blood cancer,” exclaimed the study’s lead author, Ph.D. candidate Roman Galeev. “Our findings entail a new understanding of how the expansion of blood stem cells is controlled. Eventually, this can lead to new ways of affecting the process, either to prevent the development of cancer or to expand the stem cells for transplant.”