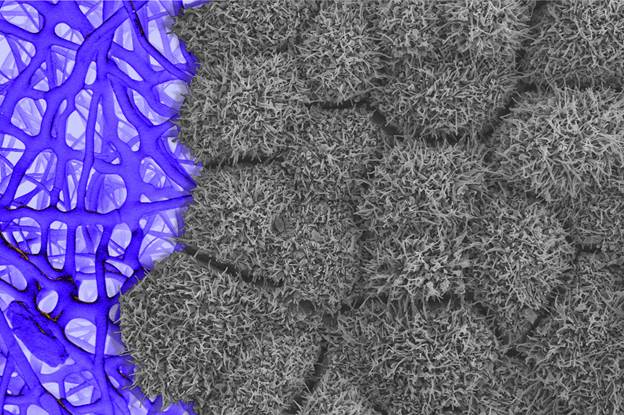
![Human iPSC-derived RPE cells (right, micrograph), here grown on a scaffold (left, artist rendered). The microvilli, or little projections on the surface of each RPE cell, communicate with the photoreceptors in the eye, an essential interaction for sight. [Nathan Hotaling (NIST); Vladimir Khristov and Kapil Bharti (NEI)]](https://genengnews.com/wp-content/uploads/2018/08/Human_iPSC_1027146238321126-1.jpg)
Human iPSC-derived RPE cells (right, micrograph), here grown on a scaffold (left, artist rendered). The microvilli, or little projections on the surface of each RPE cell, communicate with the photoreceptors in the eye, an essential interaction for sight. [Nathan Hotaling (NIST); Vladimir Khristov and Kapil Bharti (NEI)]
Cellular Dynamics International (CDI) has been awarded a $1.2 million contract from the National Eye Institute (NEI), a division of the National Institutes of Health (NIH), to manufacture clinically compatible induced pluripotent stem cells (iPSCs) and iPSC-derived human retinal pigment epithelial (RPE) cells. These cells will be manufactured from individuals suffering from dry age-related macular degeneration (AMD) and will be used for IND-enabling studies.
CDI will reprogram skin and blood samples from individuals with AMD to create clinically compatible, autologous iPSCs. These cells are intended to reduce the risk of transplant rejection. Researchers at NEI will use the RPE cells that CDI manufactures for preclinical studies in preparation for a clinical trial that will test these cells in patients with dry AMD.
“CDI has a unique ability to consistently manufacture large numbers of iPSCs and human cells to very tight specifications, including under cGMP conditions. CDI’s reprogramming platform enables us to develop autologous iPSC-based therapies, which would be a first in the U.S. We look forward to working with the NEI to successfully complete this work and progress through Phase I clinical development,” Chris Parker, chief commercial officer of CDI, said in a statement.
Two months ago, CDI received Patent No. 8,815,585 covering the automated production of human pluripotent stem cells, including iPSCs. At the time, Bob Palay, CEO of CDI, was quoted as saying, “We believe that automation is critical to both large-scale manufacture of human stem cells and differentiated cell types, and their application, especially in cellular therapeutics. The issuance of this patent helps assure our customers that CDI will be able to provide them access to manufactured human cells in the quantity, quality, purity and diversity that meets their drug discovery, stem cell banking and cell therapy research and development needs.”