April 15, 2010 (Vol. 30, No. 8)
Designing Proper Procedures to Protect Both Potential Patients and Cell-Line Production
The growing significance of biotherapeutics, particularly monoclonal antibodies (mAbs), assures that increased demand for virus-safety products and services will continue, despite the trend toward a more risk-based approach to virus removal and inactivation.
Virus safety is one component of world-wide regulatory guidelines requiring biomanufacturers to demonstrate that their manufacturing processes remove or inactivate known contaminants, among them viruses, TSEs, DNA, mycoplasma, endotoxins, and bacteria.
Virus safety encompasses two loosely related contamination issues. The better-known involves viruses that could potentially infect human patients after a product hits the market. The other focuses on viruses that are noninfective to humans, but that adversely affect the production cells or organisms.
In June 2009, Genzyme closed down production of its Fabrazyme and Cerezyme enzyme products at a Massachusetts production facility due to infection of CHO production cells with Vesivirus 2117. This was the second time this virus struck Genzyme production facilities, the first being in 2008 at its Geel, Belgium, manufacturing facility.
Vesivirus does not cause human infection but rather interferes with the growth of CHO cells. The company traced the virus to a nutrient additive, and the problem was subsequently resolved.
Genzyme’s contamination problems have led to renewed interest in contaminating viruses, particularly Vesivirus. In February, Life Technologies introduced a rapid (five-hour) molecular test for detecting Vesivirus 2117. The ViralSEQ™ detection kit uses bead-based PCR to isolate viral RNA from process samples and includes a positive control to reduce the possibility of a false positive.
Compared with older infectivity assays, PCR and its variants enable much more rapid, accurate evaluation of downstream steps for their ability to remove viruses. Bioreliance, a biosafety services company, has validated qPCR for the most common viruses affecting bioprocesses and routinely uses the technique in viral clearance studies it runs for clients. The technique is particularly effective for calculating the partitioning of virus and product after chromatography steps.
Bacteriophages, or phages, represent another significant viral contaminant that can wreck a fermentation. Unlike Vesivirus, bacteriophages infect bacteria (for example E. coli, Bacillus, or Pseudomona fermentations) and not mammalian cells, but similarly phages do not cause human disease.
“Bacteriophages are the most numerous organisms on earth,” notes Marcin Los, Ph.D., CEO of Phage Consultants, which consults and performs studies on both common and rare viral contaminants. “All production bacteria are vulnerable because phages are so prevalent in the environment. A single virus particle can destroy a fermentation.”
Perhaps the most vexing attribute of phages is their persistence, which is in good part due to their huge numbers. It is not uncommon for several phages to colonize a manufacturing facility, but one strain usually predominates. Once a bacteriophage infection is detected, preventing subsequent contaminations is difficult. Dr. Los says, “The goal after confirming infection is to prevent phages from becoming a persistent problem.”
Positively identifying bacteriophages and excluding other potential pathogenic contaminants is critical. Phage Consultants employ qPCR-based tests to detect bacteriophages and other viral pathogens. Rapid, reliable methods are essential with bacterial fermentations that tend to be shorter than mammalian cell cultures.
It is sometimes possible to recover product from a phage-infected fermentation, but this should never be done if it increases the likelihood that viruses will survive and continue to colonize the plant. Dr. Los adds, “Each liter of an infected fermentation may hold up to 1013 phage particles.” Phages spread quite easily, so in most cases it is more prudent to shut down the fermentation, kill the viruses and infected cells, take appropriate containment and decontamination measures, and start again.
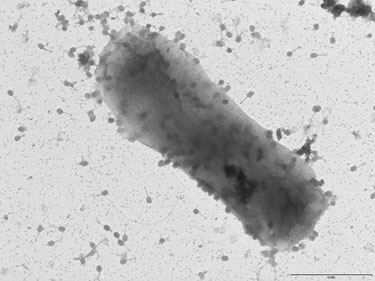
While they do not infect humans, bacteriophages can destroy a bacterial fermentation. (Phage Consultants)
Preventing Human Infection
Depending on the process, virus removal (which includes both clearance and inactivation) can employ the normal purification operations plus a final virus filtration step, with or without inactivation. For example during mAb purification the first step, a protein A capture column, is conducted at a pH low enough to inactivate many viruses.
“Depending on how long product is maintained at low pH, protein A capture is a very good step to study because it can be quite effective and very robust for inactivating enveloped viruses,” observes John Bray, Ph.D., global business development manager for clearance services at the Bioreliance Glasgow facility. Other chromatography steps (especially anion/cation exchange) may also provide several log reductions in virus concentrations, depending on the media’s ability to retain viruses and the salt concentrations, Dr. Bray explains.
That many downstream unit operations used routinely during bioproduction remove or inactivate viruses is good news. The hurdle for bioprocessors is that clearance and/or inactivation by means of these steps must be demonstrated, quantified, and shown to be robust. Recently an online tool has emerged for selecting separations tools that help achieve both product purity and virus safety.
FDA’s Office of Biotechnology Products now maintains a database on viral-clearance unit operations for the production of mAbs. The viral-clearance database, which focuses on retroviruses and parvoviruses, holds clearance data from regulatory submissions for various types of chromatography (protein A, ion exchange), low-pH inactivation methods, and virus filtration.
The best time during a product’s development life cycle to conduct virus-safety studies is a matter of debate. The two competing philosophies can be termed “early” and “late.” The more traditional late approach, which evolves from the mandate to “fail early,” argues that expending resources on an early-stage molecule that is likely to fail is wasteful. The emphasis in the late approach is toward product yield and purity, and to worry about viral clearance only when approval seems likely.
Through the early approach, developers optimize (or evaluate) each production step for viral clearance as the steps are implemented. This involves investing in virus-safety studies long before molecules enter clinical trials.
As Dr. Bray notes, the early approach has its benefits. “You know that by the time you enter early-phase clinical trials that steps are in place that provide significant clearance.” The other way, if a virus problem surfaces identifying the cause and remedying it entails significant cost and delays. For example, processors may need to insert an inactivation or filtration step that was not part of the original process. “There’s definitely a cost implication for investing this time early in process development,” Dr. Bray says, “but it saves significantly at the end because you don’t have to go back and re-evaluate the process.”

Virosart CV is an integral part of Sartorius’s 3-step viral clearance technology.
Risk-Based Approach
“The virus safety industry is reactive,” notes Andy Bailey, Ph.D., CEO of Virusure. “It tends not to address an issue until it really presents itself.” As examples, Dr. Bailey mentions experience at Genentech during the 1990s with a minute virus of mice (MVM), a parvovirus that has become arguably the number-one suspect for CHO-based processes.
Parvovirus, a nonenveloped single-stranded rodent virus discovered in 1975, represents the gold standard for removal/inactivation. Parvoviruses are among the smallest viruses known and withstand harsh pH, high temperature, and solvent treatment. Removal is typically achieved using nanofiltration. Several companies manufacture nanofiltration products suitable for parvovirus removal, including Millipore (Viresolve PRO) and Sartorius Stedim Biotech (Virosart CPV).
Despite the retrospective status quo with respect to virus safety, biomanufacturers have, in recent years, begun to shift toward a more risk-based approach to virus safety. To some degree this has reduced the regulatory burdens with respect to viral clearance. “In the past there wasn’t too much thought devoted to whether virus safety tests made sense or not,” says Dr. Bailey. “Now the authorities are much more open to discuss options if you can make a valid argument for replacing one test with another.”

Millipore’s Viresolve PRO is a nanofiltration product suitable for parvovirus removal.
New European guidelines for virus safety make it easier for manufacturers to reduce the number of virus-safety tests required for commencing human testing. “The basis for that new guideline was risk management,” Dr. Bailey observes. “The question was, does it make sense to conduct all these tests, and do they actually enhance product safety, when drugs are still at the developmental stage?”
Previously, EMEA required that companies about to enter Phase III submit clearance data on up to four viruses. This has been reduced to two viruses (typically murine leukemia virus and MVM) whose removal is orthogonal or complementary. “If you can remove those viruses, your process will also remove other model viruses that previously would have been mandated for testing,” Dr. Bailey says.
Harmonization of regulations between the U.S. FDA and Europe’s EMEA with respect to virus safety have also been a positive step, notes Franz Nothelfer, associate director of purification development at Boehringer Ingelheim.

Virosart® CPV is an integral part of Sartorius Stedim Biotech’s three-step viral clearance technology platform that includes virus filtration, inactivation, and adsorption.



