March 15, 2012 (Vol. 32, No. 6)
Canada-Based Medicago Opens U.S. Facility to Exploit Its Influenza Vaccine Production Method
Medicago says it’s improving the production of influenza vaccines in two ways: The firm starts with virus-like particles (VLPs) to generate a vaccine, and then it manufactures large quantities of a vaccine in the leaves of tobacco plants. Its technology platform is readily adaptable for the mass production of seasonal and pandemic vaccines, as well as biosimilars and other types of proteins.
Medicago’s manufacturing platform uses Nicotiana benthamiana, also known as Australian tobacco. In 1999, Louis-Phillippe Vezina, Ph.D., co-founded Medicago to explore the manufacturing of proteins in alfalfa plants. Dr. Vezina, CSO, named the company Medicago after the Latin name for alfalfa. Company researchers later discovered that tobacco produces higher yields of VLP-based vaccines than alfalfa.
Headquartered in Quebec City, Medicago recently opened Medicago USA in Research Triangle Park, NC, to manufacture affordable VLP-based vaccines. The 97,000-square-foot facility has the capacity to produce 40 million doses of seasonal influenza vaccine or 120 million doses of a pandemic influenza vaccine, the firm claims.
The U.S. Defense Advanced Research Projects Agency (DARPA) funded Medicago to show the scalable manufacturing of its plant-expressed VLP vaccines. Medicago built the new facility in response to this challenge. In Quebec City, Medicago operates a 24,000-square-foot facility for R&D and production that includes a biosafety level 2 greenhouse and a cGMP-compliant extraction and purification unit.
Medicago USA is gearing up to conduct a rapid-fire test for DARPA. “We will attempt to manufacture 10 million doses in one month of the H1N1 influenza vaccine,” says Andy Sheldon, president and CEO. The DARPA project is part of the Blue Angel influenza vaccine rapid response project to identify new ways to produce large amounts of high-quality vaccines in less than three months to respond to emerging biological threats.
Medicago’s manufacturing platform uses Nicotiana benthamiana, which, it says, allows the bulk production of vaccines.
Advantages of VLPs and Tobacco
VLPs are lipid shells studded with short strands of proteins specific to an infectious disease that are readily recognized by the human immune system. VLPs look like a virus, but they lack core genetic material, making them noninfectious and unable to replicate. Only the genetic sequence of a virus or bacterium is needed to make a VLP-based vaccine.
“Our technology works well with wild-type viruses. We don’t need to wait for seasonal strains to mutate, so we can produce vaccines more quickly,” says Sheldon.
VLP-based vaccines provide protection against different strains of a virus other than those for which the vaccine was formulated. Medicago’s technology not only creates vaccines that exactly match a specific seasonal or pandemic strain in circulation, but they also offer strong cross-protection against other subtypes of influenza.
Tobacco plants offer an alternative to making vaccines in egg-based systems. In the last swine flu pandemic, it took up to six months for vaccines to reach the market. “In a serious pandemic, that would be too long,” says Sheldon. In contrast, Medicago produced its swine flu vaccine just 19 days after receiving the genetic sequence.
Unlike E. coli, yeast, and other unicellular organisms used to express proteins, tobacco plants are more complex and can produce very complex molecules. Additionally, the cultivation, scale-up, and maintenance costs of manufacturing VLP-based vaccines in tobacco are relatively low, and plants in general are not plagued by viral contaminants from animal sources.
How It Works
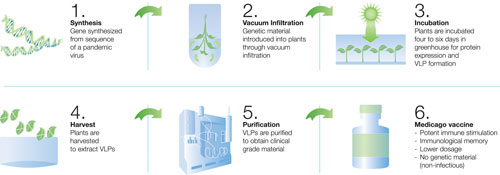
Medicago’s overall process is a transient system that does not modify the plant genome. Researchers place a viral genetic sequence into a plasmid, which they clone and insert into Agrobacterium, which carries the genetic signal into a plant. Leaves of mature tobacco plants are placed in a solution of Agrobacterium, then a vacuum compresses the leaves to expel air from the plant cells.
When the vacuum is broken, Agrobacterium infiltrates the air spaces and transfers its genetic material for vaccine production. The plants are incubated in a greenhouse for about six days to allow the expression and formation of VLPs.
Incorporation of the genetic material switches on cellular machinery that produces large amounts of proteins. The manufactured protein embedded into VLPs floats between the plasma membrance and cell wall. A patented biochemical process releases the protein, and VLPs are extracted, harvested, and purified to obtain clinical-grade material.
Clinical Programs
In a Phase II part B trial of its H5N1 avian influenza, Medicago found that the VLP vaccine induced a solid immune response and was safe and well tolerated. In the earlier Phase II part A trial, 135 volunteers received varying dosages or a placebo to determine the optimal dose. Two doses were given 21 days apart, and 20 micrograms proved the best dosage. The part B study gave an additional 120 volunteers the optimal dosage or the placebo.
The results also showed that older and younger volunteers responded similarly to the H5N1 vaccine, an advantage over other vaccine technologies. Among vaccinated volunteers who were 18 to 49 years old, 77% developed an immune response against the H5N1 virus, as did 76% of vaccinated volunteers older than age 50 years.
The H5N1 vaccine is intended as a pandemic product in case the virus mutates and becomes capable of human-to-human passage. If that occurs, “we could create stockpiles of vaccine around the world,” says Sheldon.
Medicago has other undisclosed vaccine candidates in its pipeline. It also plans to make monoclonal antibodies and biosimilars. Ten monoclonal antibodies have been efficiently expressed in tobacco.
“Many monoclonal antibodies are coming off patent by 2020. This is a real opportunity for us,” says Sheldon. However, biosimilars and monoclonal antibodies are more difficult and costly to manufacture than vaccines, “so we will be looking for partners to move forward,” says Sheldon.
Adapting plants as production systems has evolved slowly. “Like the early days of cell culture, it took time to get it right. But our time has come, and we have the opportunity to benefit human health,” says Sheldon.