November 1, 2009 (Vol. 29, No. 19)
Established System Seeks to Simplify Screening and Selection of Mammalian Cell Lines
Isolation of candidate mammalian cell clones by limiting dilution, ring cloning, or simple manual collection of colonies is a time-consuming, resource-intensive, and costly procedure that is prone to cross contamination of cells and user error. One major disadvantage commonly encountered is the difficulty in establishing monoclonality—an essential step in the production of therapeutic proteins and antibodies.
To overcome many of the drawbacks typically associated with isolating choice mammalian cell clones, Genetix has developed the ClonePix™ FL system for screening and selection of secretory cell lines. The utility of ClonePix FL lies in its ability to visualize and quantify proteins secreted from thousands of clones in situ, and to select and accurately isolate only the highest value secretors, shortening time scales and simplifying downstream culture.
The ClonePix FL solution has been validated for specific detection of secreted antibodies and monomeric proteins, cell surface proteins, and intrinsic GFP fusion proteins. It is compatible with a range of cell types including hybridoma, myeloma, HEK293, and both suspension-adapted and adherent CHO cells.
Workflow
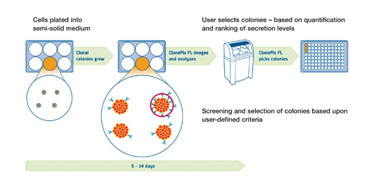
A fundamental initial step in the workflow (Figure 1) is the culturing of mammalian cell lines in semisolid media, such that cells form discrete colonies, each originating from a single parent cell. Heterogeneous populations of thousands of cells are grown into clonal colonies in either one-well or six-well microplates. The cells are then screened rapidly, using white light to detect the colonies and fluorescence to quantify secreted protein in situ.
The secreted protein-detection assay requires the secreted product to be immobilized around the colony using a fluorescent detection probe. Dedicated ClonePix FL software quantifies the fluorescence associated with each colony and drives the automated picking of only the most desirable clones into 96-well destination plates. Figure 2 shows example images that identify the highest producing clones. A variety of different proteins can be detected and isolated simultaneously by using detection agents labeled with different fluorophores (multiplexing).
For hybridoma fusions, clones can be selected based on antigen-specificity by additionally adding antigen as the detection probe; this can be directly conjugated with fluorescence or via a secondary detection agent. Plates of picked colonies are then grown to confluence in preparation for clone expansion and scale-up.
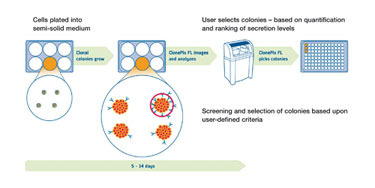
Figure 1. ClonePix FL workflow
Assessing Monoclonality: Statistics
Clonality of hybridomas collected from semisolid medium was previously evaluated and it was concluded that, at appropriate seeding density, the probability of coincidence (nonclonality) is 4%. Genetix has also generated a statistical calculation that shows a correlation between the probability of monoclonality and seeding density/size of colony.
In a typical experiment, CHO cells are plated into semisolid medium to produce 25 colony outgrowths per 35 mm diameter well (i.e., a six-well microplate well). At day 14, the colonies are around 0.75mm in diameter. Using the formula (0.25 x Pi x (0.75+0.75)2 x (n-1)) / (0.25 x Pi x 352), where colony number n=25, the probability of coincidence is 4.4%, thus the probability of monoclonality for one round of cloning should be 95.6%. This is very close to the value determined previously.
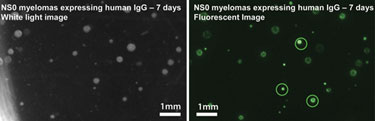
Figure 2. Fluorescent detection of a secreted IgG from an NSO myeloma cell line
Assessing Monoclonality: Experimentation
To test the statistical theory experimentally, we combined two IgG-secreting hybridoma cell lines, one secreting IgG1 and the other secreting IgG2a. Colonies were grown in CloneMatrix-based semi-solid media in the presence of fluorescent-labeled isotype-specific antibodies against the IgG1 and IgG2a. AlexaFluor 488-conjugated anti-IgG1 was visualized in the FITC channel of ClonePix FL, and AlexaFluor546-conjugated anti-IgG2a was visualized in the rhodamine channel. Representative images are shown in Figure 3.
The colonies were analyzed using ClonePix FL software and differentially picked according to whether they were shown to have high exterior mean intensity of FITC with low Rhodamine intensity (for IgG1 secretors), or low FITC with high Rhodamine (for IgG2a secretors).
A total of 312 colonies (156 of each type) were picked and subsequently grown in 96-well plates for four days. The conditioned media were then assessed for IgG1 and IgG2a using isotype-specific ELISA assays (Bethyl Laboratories).
Cells with either poor outgrowth or a negative ELISA for both IgG1 and IgG2a were excluded. The results showed that, of the 143 colonies that were picked based on IgG1-specific fluorescence, only one showed a low level of IgG2a (clonality=99.3%). Of 81 IgG2a colonies picked based on IgG2a-specific fluorescence, three were heterogeneous (clonality=96.4%). Monoclonality for the complete data set was calculated as 98.25%.
This experimental data validates the statistical evaluation that clonality after a single round of picking on the ClonePix FL system is >95.6% when picking larger-sized colonies (0.75mm diameter). Using the same formula, it can be calculated that by picking at an earlier time point (when colonies are only 0.35 mm in diameter), the probability of monoclonality is increased to 99.0%. In addition, performing a second round of cloning (replating the picked clones and repicking) increases the probability of monoclonality to 99.9%.
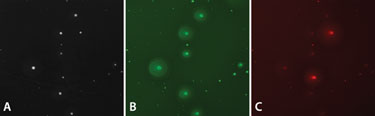
Figure 3. ClonePix FL images from the mixed hybridoma experiment
Summary
The ClonePix FL system has been demonstrated as a powerful tool for rapid screening and isolation of secretory mammalian cell lines typically used in the production of therapeutic proteins and research monoclonal antibodies. The system increases overall productivity, leaving more time to better characterize antibodies and begin new projects. The data presented in this tutorial supports the idea that picking colonies of mammalian cells from semisolid medium is an effective means to isolate clonal cell lines.
Omee Ahmed is student lab technician, Julian F. Burke, Ph.D., is CSO at Genetix, Christopher J. Mann, Ph.D. ([email protected]), is senior application support specialist, Sky Jiang is research manager, Kerensa J. Klottrup, Ph.D., is senior scientist, and Natalie Smithers, Ph.D., is a postdoctoral scientist. Web: www.genetix.com.