October 15, 2015 (Vol. 35, No. 18)
Revamped Allograft and Innovative Xenograft Models Can Reduce the Risks of Late-Stage Clinical Trials and Increase the Odds of Translational Success
Precision medicine is all about the two T’s—targets and treatments—targets that emerge from analyses of patient-specific information, and treatments that hit these targets. Both T’s, however, pose difficulties. All too often, insights and drug candidates originating in precision medicine fail to translate into clinical practice.
This reality prompted sober comments at the most recent event in the Tumor Models series organized by Hanson Wade. “One of the current problems with identifying new targets for precision medicine is identifying biologically meaningful targets likely to translate to the clinic,” complained Nicola McCarthy, Ph.D., oncology program manager, Horizon Discovery.
That pretty much sums up the difficulty with the first of the T’s. Now, what’s so hard about the second one? “It’s estimated that about 94–96% of drugs making it through preclinical testing stages to clinical phases ultimately fail in the clinic as a result of poor efficacy or poor safety,” lamented Leon Hall, Ph.D., senior director, global scientific development, Taconic Biosciences.
Drs. McCarthy and Hall were among presenters at Tumor Models Summit Boston 2015. Meeting highlights, discussed herein, included in vitro approaches, such as the powerful gene-editing technology known as CRISPR, as well as in vivo approaches, such as mouse models for immuno-oncology strategies.
Dr. Hall spoke to the common goal: “We believe, as others do, that by improving the ability of mouse models to mimic human biology, the models will better predict drug responses in the human patient.”
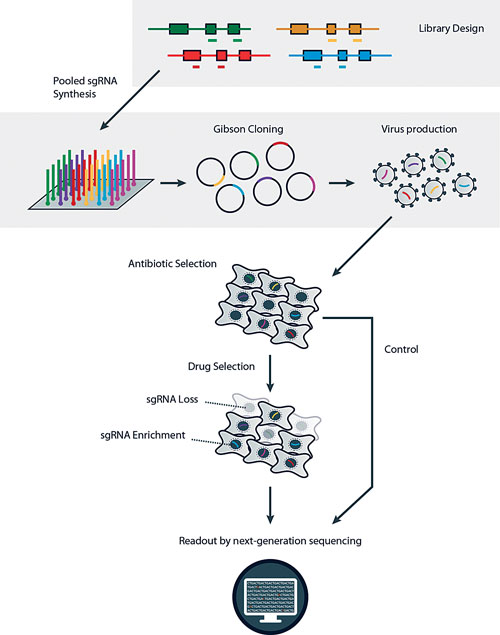
CRISPR-Cas9 sgRNA in vitro screens can be used to look for genes that when lost induce resistance to a drug (positive screen) or increase sensitivity to a drug (negative screen). The technology might also be a powerful tool for target ID screens in vivo, and looks set to aid in our understanding of tumor development and progression in animal models. [Horizon Discovery]
CRISPR-Modified Screens
“Cell lines are such fantastic tools when you have very targeted mechanistic questions,” exclaimed Tommy Broudy, Ph.D., general manager, Crown Bioscience. In collaboration with Horizon Discovery, Crown Bioscience has developed isogenic cancer models for in vivo compound screening. For example, using Horizon’s rAAV-based Genesis™ gene-editing platform, the companies introduced mutations to genes such as KRAS, PIK3CA, PTEN, IDH1 and IDH2, and p53.
Expanding its technological capabilities, Horizon Discovery recently added CRISPR-Cas9 single-guide RNA (sgRNA) screening capabilities to a tool collection that already included siRNA cell-line screens.
Directed by sgRNAs, Cas9 nucleases cut at specific locations in the genome. “Unlike siRNA screens, CRISPR-Cas9 sgRNA screens enable complete loss of a particular protein,” said Dr. McCarthy. “This can be informative in terms of identifying and validating targets for which a phenotype of interest is evident only on complete loss of protein expression.” Such targets, Dr. McCarthy emphasized, can be missed by siRNA screens because full depletion of a target often does not occur consistently between siRNAs.
Horizon employs CRISPR-Cas9 screens to identify targets amenable to a synthetic lethality approach. Synthetic lethality arises if two conditions are met: 1) mutation of either of two genes is compatible with viability, and 2) mutations in both genes result in cell death. Potential “synthetic lethal” targets identified by Horizon include specific common mutations occurring in colorectal cancer, such as p53 and KRAS.
The screens involve putting an sgRNA library targeting thousands of genes into a cancer cell population, aiming for one sgRNA infection per cell so multiple genes aren’t cut in any one cell.
The sgRNA population is exposed to a target drug of interest, perhaps one being considered for combination drug therapy. Next-generation sequencing identifies baseline and survivor sgRNAs.
The sgRNAs lost during treatment are potential synthetic lethal targets, suggesting that the corresponding knocked out gene is essential for the viability of a cancer cell line.
Identifying targets in this way can be “like looking for a needle in a haystack,” asserted Dr. McCarthy. “Difficult cellular changes that once took a long time to engineer, however, are now generally much more straightforward with CRISPR-Cas9 technology.”
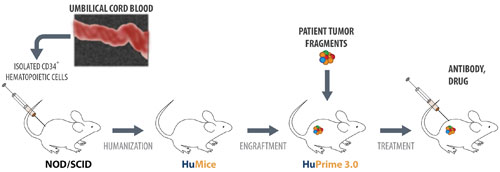
Crown Bioscience is developing research platforms for preclinical evaluation of drugs that harness the human immune system to fight human tumors. For example, the company provides HuPrime® 3.0, a patient-derived xenograft model. It is based on humanized mice, HuMice™, immunocompromised mice that have been inoculated with human hematopoietic cells (from cord blood stem cells).
From Cell Lines to Mouse Models
“We certainly run many cell-line studies, but we have slightly more translational questions when trying to understand or predict what may happen in the clinic, in the patient population,” informed Dr. Broudy. “Cell lines are not the best tools for that. For translational questions, you want to have models that are as clinically relevant as possible, models that maintain the heterogeneity of cancer and genomic equivalency to a patient tumor.”
Mouse models fit the bill. Today’s models are able to accommodate ever-evolving scientific and precision/personalized medicine missions, which include precisely editing the genome and harnessing the immune system to fight cancer.
Case in point: Dr. McCarthy said that potential targets identified from siRNA or CRISPR-Cas9 sgRNA cell-line gene-editing screens are validated through more complex in vitro models such as 3D culture. Targets potentially involved in modulating tumor immune responses might advance to in vivo models, such as syngeneic mouse models, for further validation.
Transplantation Model Options
In general, transplantation mouse models come in two types: allograft (mouse on mouse) and xenograft (human on mouse). An allograft mouse tumor system, which typically consists of a mouse cell line on a mouse immune system, is known as a syngeneic model. Xenograft systems include cell-line xenograft models and patient-derived xenograft (PDX) models. Yet another xenograft system is the humanized hybrid.
“In a humanized hyrid,” explained Dr. Broudy, “a human tumor or a tumor cell line is engrafted into a mouse in which a human immune system has been reconstituted.” Models of this sort are also called humanized mice. Such models, Dr. Hall added, are meant to better emulate human biology.
Crown, the premier PDX company, has almost 1,600 PDX models. Their expanding collection represents a global population including models established from American, Asian, and European patients. “And that accounts for the incredible diversity of models our clients, pharma drug developers, can select from,” declared Dr. Broudy.
“Syngeneic” means genetically similar such as putting a tumor that originated in a C57 black mouse into a different C57 black mouse, meaning the animal has an intact immune system. With increasing efforts to harness the immune system to treat cancer, syngeneics have a big role in immuno-oncology drug development.
“The tool set just keeps getting bigger and bigger,” Dr. Broudy insisted. “It is allowing us to ask lots of different questions.” A complementary sentiment is expressed by Walter Ausserer, Ph.D., associate general manager of clinical and in vivo services at The Jackson Laboratory (JAX): “I think the science is still young enough that you need to be using every tool you can.”
Immuno-Oncology Modeling
Immune-inhibitory pathways (immune checkpoints) are essential for maintaining self-tolerance and modulating immune responses. Immune T cells have a natural ability to destroy cancer cells. This capability of T cells can be inhibited by tumors, which develop immune suppressor mechanisms and thereby escape immune surveillance, gaining the capacity for uncontrolled growth. For example, cells in the tumor microenvironment or a tumor itself may express high levels of immune checkpoint proteins such as CTLA4 or the programmed cell death PD-1 ligand, respectively, which negatively regulate T-cell activation.
“In immuno-oncology, we are trying to activate a patient’s immune system to recognize and attack that patient’s tumor,” said Dr. Ausserer. Immune checkpoint inhibitor drugs that counteract tumor immune suppression mechanisms and promote immune system activation represent a key focus of immuno-oncology drug development.
The co-engrafted NOD scid gamma (NSG) humanized mouse developed by JAX is “grafted with both the human immune system and human tumors,” Dr. Ausserer continued. “It is one of the first in vivo model for studying interactions between human immune system cells and human tumors.
“This area of immuno-oncology is uniquely personal, akin to personalized medicine. You’re trying to coax an individual patient’s immune system to recognize and attack that same patient’s tumor.”
Dr. Broudy concurred. “We’ve been putting significant effort into extending the utility of PDX and cell-line xenograft mouse models for immuno-oncology drug development, including utilizing humanized mice. The truth is, if you don’t have a human immune system in the mix and are using only typical human patient-derived tumor xenograft models, there’s not a whole lot of value for immuno-oncology.”
Another angle was emphasized by Aidan Synnott, Ph.D., site director at Charles River Laboratories. “With the advent of immunotherapies,” he said, “we’ve taken a look at the very old syngeneic mouse models and basically revitalized them.”
Syngeneic models have been used since the 1960s. In the 1990s and 2000s, however, people started moving toward xenograft models. According to Dr. Synnott, this trend gained momentum when immunodeficient mouse technology became available.
The real impetus to immunotherapy came with the 2011 approval of Bristol-Myers Squibb’s Yervoy (ipilimumab), the first checkpoint inhibitor. This therapeutic, which blocks CTLA4, led to widespread recognition that syngeneics offer advantages over conventional human-on-mouse PDX models for immuno-oncology studies because of their functional immune systems.
Indeed, Merck, Amgen, and other pharmaceutical companies used syngenic mouse models in developing their approved therapies, recalled Dr. Synnott. Syngeneic mouse tumor models of B16-F10 melanoma and M38 colon adenocarcinoma were both used in developing Amgen’s leukemia drug Blinatumomab and Merck’s melanoma drug Keytruda.

According to Charles River Laboratories, immuno-oncology development may be ill-served by con- ventional xenograft models. Such models may lack relevance because they rely on immuno-compromised animals. Syngeneic mouse models, however, represent an attractive alternative. They can show how cancer therapies perform in the presence of a functional immune system.
Mechanistic Insights
However, mechanisms underlying drug efficacy and efficacy endpoints still remain to be fully elucidated and defined. So Charles River is running studies to do exactly that. For example, the company employs flow cytometry to assess drug effects on the balance of different types of immune cells with antibodies.
“In some of our syngeneic models following checkpoint inhibitor treatment, the number of CD4-positive T cells will go down,” explained Dr. Synnott. “However, the number of CD8 effector T cells that actually attack the cancer will go up.
“We help our clients understand how their drug is actually having an effect. You don’t just tell them, ‘Your tumor shrank, so your therapy works.’ You tell them the tumor shrank because it enhanced a certain subset of immune cells which then presumably attacked the tumor and shrank it.”
Mighty Human Mouse Models
Taconic Biosciences’ portfolio of precision research models consists of genetically engineered humanized mice carrying human genes, and mice engrafted with human cells and tissues. “We work very closely with Japan’s Central Institute for Experimental Animals,” said Dr. Hall. “They focus on the genetic development of what we call super-immunodeficient mice that can be readily engrafted with foreign cells and tissues. These become the basis of our cell- and tissue-engraftment portfolio.”
huNOG, Taconic’s primary immuno-oncology model, is a NOG (NOD/Shi-scid/IL-2Rγnull) mouse engrafted with a human immune system. It expresses a variety of human immune cell lineages including T and B cells.
Much of today’s immuno-oncology work focuses on “enhancing T-cell functionality within cancer patients to enable their T cells to activate, target the tumor, and destroy tumor cells,” stated Dr. Hall. Human huNOG T cells are functional and mimic responses seen in human patients treated with immune checkpoint inhibitory drugs. A huNOG mouse can successfully engraft a human primary tumor.
“You can treat those mice with a drug such as ipilimumab, and the human immune system will respond showing typical markers of activation seen in human patients, such as increases in T cells,” Dr. Hall continued. “Immune cells such as cytotoxic T cells infiltrate the tumor, resulting in tumor regression.”
After putting a human patient tumor on board, immune responses generated by the tumor’s presence are assessed by flow cytometry and other techniques. Both immune responses and interactions between the human immune system and human tumor are evaluated following therapeutic intervention.
Historically, pharmaceutical and biotechnology companies developed drugs for preclinical studies in mouse models that were, well, mouse specific. Due to species specificity, these drugs would not be exactly the same as the large molecule drug that would be utilized in humans.
Dr. Hall wrapped up as follows: “Being able to place a human immune system within the mouse allows investigators to utilize the same therapeutic agent in the mouse model that will move to the clinic.” As a result, more drugs should be successful once they reach clinical testing.



