January 1, 2009 (Vol. 29, No. 1)
Synthesis and Characterization: Past, Present, and Future Practices
The alleviation of pain is perhaps the greatest long-standing goal of both ancient and modern medicine. Quite recently, opiate receptors were implicated in the propagation of pain. With exquisite complimentary molecular architecture, these protein pockets tightly bind smaller endogenous molecules, setting off a cascade of intracellular events, resulting in the pain response.
This critical realization has spawned a successful strategy for pain relief; namely, that competitive blocking of opiate receptors with even more tightly bound synthetic analgesics (termed opiate antagonists) could successfully disrupt the sensation of pain.
To assist in the identification and optimization of novel and safer analgesics, the past several decades have witnessed the creation of innovative biochemical methods.
Many of the reagent tools PerkinElmer has created for drug discovery have been radioactively tagged small molecules (radioligands). They accurately and sensitively track radioactivity and consequently aid in the understanding of the interaction of these radioligands in cells associated with the biochemistry of pain. Tritium, the radioactive isotope of hydrogen, confers no structural change on the molecule and has thus been an especially useful label.
In 1973, Professor Solomon Snyder of Johns Hopkins University reported a profound technical breakthrough in the general area of drug discovery and the analgesic field in particular. Using the well-known opiate antagonist naloxone, labeled with tritium by PerkinElmer, Professor Snyder identified an opiate receptor through an efficient sensitive biochemical method that has come to be known as a receptor binding assay.
Since then, the receptor binding assay has become a valuable tool for drug discovery, and PerkinElmer has provided optimized radioactive reagents for this technology. The tritium-labeled naloxone first employed by Professor Snyder was indeed radioactive, but the degree of radioactivity incorporation in the molecule was rather low. It was quickly appreciated that higher specific activity opiate receptor radioligands would be needed to increase the assay’s sensitivity and scope, and that new tritiation methods would be required to obtain that goal.
One of the strategies we turned to was catalytic tritium dehalogenation, relying on the ability of a heterogeneous catalyst (usually palladium on some support) to efficiently replace halogens (like bromine and iodine) with tritium atoms in precursor molecules at high specific activity.
Using this technique, we have been able to tritiate many useful opiate receptor (and related) ligands including the kappa opiate receptor agonist bremazocine, the fluorine-containing analgesics brifentanil and ocfentanil, and the nonpeptide substance P antagonist CP-96,345 in close collaboration with Pfizer scientists.
Another way to achieve high specific activity tritiation is by the design and synthesis of unsaturated precursor molecules that could also be catalytically tritiated, providing the desired radioligand at even higher specific activity than possible with catalytic tritium dehalogenation. Indeed, it was by using this technique that we soon revisited naloxone (Figure 1) and tritium-labeled it at far higher specific activity, employing a Lindlar catalyst reduction of a synthetic alkyne precursor.
Furthermore, this new and higher specific activity version of [3H] naloxone (Figure 1) had tritium attached in a specific and far more stable location than that previously employed by Professor Snyder in his early work. Utilizing this improved radioligand, we were able to also synthesize the irreversible dimer analogue [3H] naloxonazine (Figure 2) by means of exacting small-scale radiochemistry. Another example of successful implementation of this radiolabeling method was the tritium labeling of lofentanil, an extremely potent analgesic, with partners at Janssen. Using this technology, PerkinElmer has tritiated literally scores of valuable analgesic compounds at high specific activity.
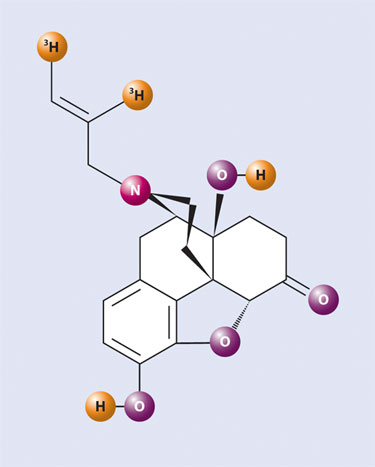
Figure 1. [3H] Naloxone
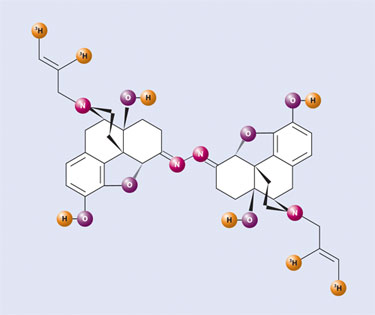
Figure 2. [3H] Naloxonazine
Tritiation Process
The synthetic process to radiolabel with tritium is a multistep process. The catalyst is added to a glass flask with special sidearms and a stir bar. The appropriate precursor, a halogenated or unsaturated compound (1–50 mg), is dissolved in a minimum amount of solvent (1–2 mL), and then is also added to the flask. The flask is attached to a stainless steel vacuum line. The flask is partially evacuated, lowered into liquid nitrogen and then fully evacuated. Tritium gas is introduced to the flask to the appropriate pressure. The reaction is stirred for the designated amount of time, and pressure is monitored to track incorporation of tritium into the compound.
Once the reaction is complete, the flask is again evacuated to remove excess tritium gas. Additional solvent is reintroduced, and the flask contents are filtered in line and transferred to a receiving flask. Labile tritium is removed by repeated additions of protic solvents (e.g., water, methanol) and repeated evacuations to dryness. The product is then dissolved in a solvent, ready for purification.
PerkinElmer has developed an automated tritiation line to handle this process. This control improves safety, consistency, and reduces tritiated waste.
A final tritiation technique that greatly expanded the limits of specific activity was based on the fact that many opiate-receptor compounds possess methyl groups attached to heteroatoms like nitrogen or oxygen. Exploiting this structural circumstance facilitated removal of these methyl groups from compounds, yielding N or O-desmethyl precursor analogues of them.
As a result, methyl groups labeled at nearly theoretically high specific activity with tritium using either [3H] methyl iodide or, more recently [3H] methyl nosylate, can be reattached. Products of this methodology are compounds like [N-methyl-3H] morphine (Figure 3) and [N-methyl-3H] dihydromorphine.
Early on, PerkinElmer observed that some of these radioligands were so highly radioactive that they manifested the curious behavior of isotopic fractionation on HPLC. Awareness of this intriguing chromatographic property was critical to their purification and characterization.
The ongoing effort to tritium label opiate-receptor ligands has also prompted us to utilize and extend the boundaries of instrumentation to prepare and characterize these substances. Long ago, we recognized the importance and power of tritium NMR to analyze tritiated opiate ligands, and we conducted early experiments over 30 years ago. An example of this, the proton decoupled tritium NMR (CD3OD) of the semi-synthetic opiate [3H] buprenorphine, is shown in Figure 4.
The tritium NMR spectrum of this radioligand clearly corroborates that the molecule has been labeled with tritium predominantly in the buprenorphine 15 and 16 bridgehead positions as evidenced by the large sharp multiplet peaks near 2.0 and 3.0 ppm. It also interestingly reveals that this HPLC homogeneous product contains a minor amount of nonspecific tritium incorporation in other locations of the molecule as disclosed by other smaller tritium resonances.
Mass spectrometry has been indispensable in establishing product identity and even calculating the specific activity of highly tritiated opiates. With such small masses, highly tritiated substances cannot be accurately weighed to ascertain their mass for specific activity calculations, but mass spectrometry easily facilitates this measurement.
The mass spectrum of high-specific activity [N-methyl-3H] morphine, clearly documents the presence of a large radioactive (M + I) parent ion at 292.5 m/z due to the incorporation of three tritium atoms in the product by tritium N-methylation (Figure 5). Comparing the size of this dominant radioactive parent peak with that of the small amount of unlabeled morphine at 286.5 m/z allows the measurement of tritiated product specific activity to be 85.5 Ci/mmol.
Over the past several decades, significant advancement has been made in the synthesis and characterization of tritiated opiate radioligands. These days even more structurally complex and challenging opiate drug candidates are being identified. The future will demand and no doubt witness the development of newer tritiation and analytical methods to accomplish the radiolabeling of these important substances.
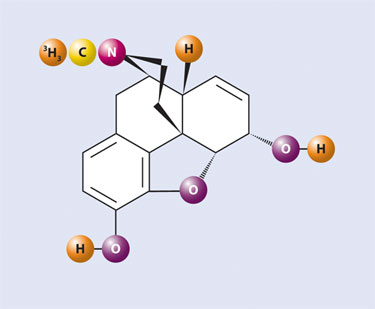
Figure 3. [3H] Morphine
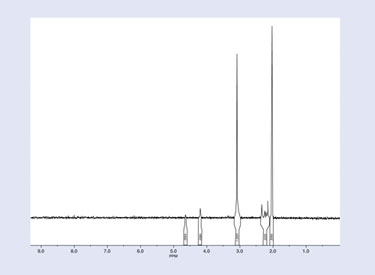
Figure 4. Proton decoupled tritium NMR of [3H] buprenorphine
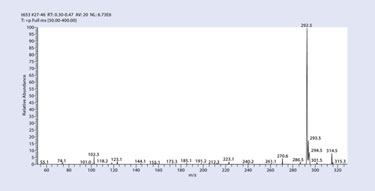
Figure 5. Mass spectrum of high specific activity [N-methyl-3H] morphine



