October 1, 2008 (Vol. 28, No. 17)
Diseases Like CKD, Cancers, and Psoriasis Can Benefit from the Blocking of CYP24 Activity
Emerging data from new research suggests that abnormally elevated CYP24 expression contributes to the pathology of certain diseases including chronic kidney disease (CKD), cancers, and psoriasis.
CYP24, a member of the cytochrome P450 enzyme superfamily, is the key enzyme involved in vitamin D catabolism. Increased levels of CYP24 decrease the effectiveness of vitamin D replacement therapies and potentially contribute to local or systemic vitamin D insufficiency. Specific inhibition of CYP24 provides a new approach to treating diseases responsive to vitamin D therapy.
Enzymes belonging to the cytochrome P450 superfamily catalyze critical biochemical reactions in the body including hydroxylations, epoxidations, oxygenations, and dehalogenations, which are needed to activate or inactivate endogenous-signaling ligands and detoxify xenobiotic compounds such as drugs. Those P450s exclusively involved in the metabolism of endogenous ligands such as fat-soluble vitamins (vitamins A and D), fatty acids, steroids, eicosanoids, and bile acids have attracted interest as potential drug targets because they play pivotal roles in modulating tissue responses to these substances and their synthetic counterparts.
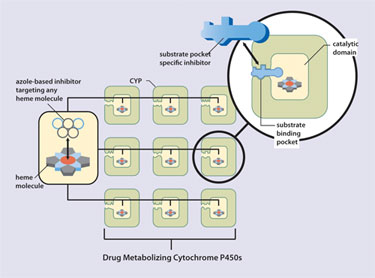
A number of P450 inhibitors have been developed that utilize functional azole groups to target the heme moiety at the enzyme’s catalytic core. Because heme resides at the core of all P450s, such inhibitors lack specificity and, consequently, can block activity of drug-metabolizing P450s, thus predisposing patients to possible drug-drug interactions (Figure 1). New vitamin D compounds targeting the CYP24 substrate binding pocket, rather than the catalytic site, allow much more potent inhibition with higher specificity.
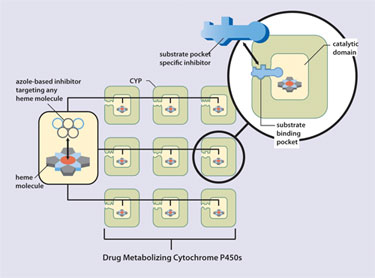
Figure 1. Ketoconazole vs. vitamin D compound for the inhibition of CYP24.
Signaling
Calcitriol (1α,25(OH)2D3), the active hormone of vitamin D3, is well known for its role in bone metabolism and calcium homeostasis.
Calcitriol synthesis involves multiple steps, beginning with the formation of the precursor vitamin D3 in the skin following ultraviolet light exposure. Vitamin D3 undergoes two sequential metabolic steps to form calcitriol. The initial step involves 25-hydroxylation in the liver by CYP27A1 to form calcifediol (25(OH)D3), followed by a second step that involves 1α-hydroxylation in the kidney by CYP27B1 to form the active hormone. CYP24 is the enzyme responsible for catabolism of calcitriol via 24-hydroxylation to generate 1,24,25-trihydroxyvitamin D3. CYP24 also catabolizes calcifediol to yield 24,25-dihydroxyvitamin D3.
In addition to its well-known effects on bone and mineral homeostasis, calcitriol has antiproliferative effects and promotes in vitro differentiation in a wide variety of cell lines. Calcitriol also induces CYP24 expression, thereby limiting its own access to VDR in target tissues because of the resulting increase in its catabolism. CYP24 inhibitors, therefore, have enormous promise in potentiating therapeutic vitamin D hormone effects.
Design of CYP24 Inhibitors
The most effective way to specifically inhibit an enzyme like CYP24 is through its substrate-binding pocket, a unique functional domain. Vitamin D metabolizing enzymes such as CYP24 use specific chemical groups such as a hydroxyl group positioned on the vitamin D scaffold to appropriately position selected molecules in the substrate-access channel.
Based on this knowledge, Cytochroma used calcitriol as the template and chemically modified either the side chain and/or the A ring to appropriately achieve highly specific and effective inhibition of CYP24 (Figure 2). The resulting compounds were screened using two different assays: an inhibition assay and a cell-based transcription activation assay.
The first assay used the Chinese Hamster V79 cells stably transfected with CYP24. In this assay, calcitriol was incubated for 40 minutes in the presence of different concentrations of compound after which time the percentage of calcitriol catabolism was measured by HPLC. Ketoconazole, known to inhibit CYP24 (among other P450s), was used as a benchmark. Potent compounds were also tested for their ability to inhibit other P450 enzymes in order to determine their specificity.
The second assay, a transcriptional activation assay, was used to determine whether the compounds had retained their ability to activate vitamin D signaling through the vitamin D receptor (VDR).
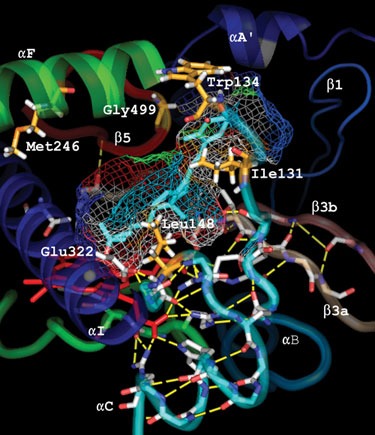
Figure 2. Compound docking in the substrate binding pocket of CYP24.
Pure Inhibitors
Using this approach, we have identified a number of pure inhibitors based on their high potency in inhibiting CYP24 activity and their low VDR transcriptional activity. The identified inhibitors (e.g., CTA091 IC50Figure 3) compared favorably to ketoconazole, which itself has a respectable IC50 in the 400–600 nM range. These inhibitors were selected because they exhibited low or no residual ability to induce CYP24 gene expression. In addition, inhibitors like CTA091 do not inhibit the closely related cytochrome P450s such as CYP27A (which metabolizes vitamin D3) or CYP27B1 (which metabolizes calcifediol).
Epidemiological studies have provided strong evidence that adequate endogenous supply of calcitriol is associated with reduced incidence of certain cancers including carcinomas of the prostate, colon, and breast.
Studies conducted both in vitro and in vivo support calcitriol’s role in regulating proliferation, differentiation, apoptosis, angiogenesis, and the metastatic processes involved in tumorigenesis.
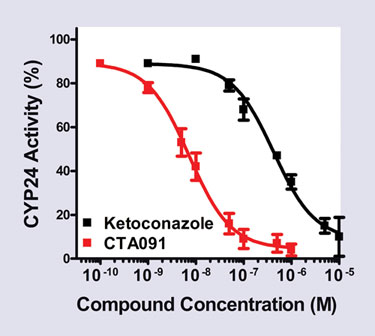
Figure 3. CYP24 inhibition by CTA091 compated to ketoconazole.
CYP24 in Oncology
Rapid catabolism of calcitriol by CYP24 is a significant barrier to the hormone’s potential therapeutic use in cancer treatment. Calcitriol upregulates CYP24 gene expression, thereby promoting its own catabolism and leading to gradual development of treatment resistance.
Interestingly, recent analyses of several human cancer tissues (colorectal, gastrointestinal, prostate, and skin) at the molecular level have demonstrated abnormally elevated CYP24 expression compared to normal tissue and a correlation between high CYP24 expression and tumorigenicity. These observations suggest that specific CYP24 inhibition may have therapeutic potential in cancer prevention and treatment. In addition, CYP24 inhibition may also enhance the efficacy and pharmacokinetic profile of calcitriol and its analogs by limiting their catabolic breakdown.
CYP24 in CKD
In CKD, secondary hyperparathyroidism frequently arises as blood levels of calcitriol decline. Blood parathyroid hormone (PTH) levels, normally controlled by calcitriol, begin to increase as the capacity of the failing kidneys to produce adequate calcitriol steadily diminishes.
Exogenous calcitriol, doxercalciferol, and paricalcitol effectively lower PTH but also induce CYP24, especially when administered by bolus intravenous injection. It is likely that elevated CYP24 plays a central role in contributing to the widespread vitamin D insufficiency observed in CKD patients, a problem that has recently been linked to increased patient mortality.
CYP24 Inhibitors in the Clinic
Pure CYP24 inhibitors can provide clinicians in several disciplines with new tools to use directly or in conjunction with vitamin D replacement therapies to treat vitamin D responsive diseases. Blocking CYP24 activity in hyperproliferative diseases like cancer and psoriasis can enable optimal penetration of target tissue with vitamin D hormones. Limiting CYP24 activity in a progressive disease like CKD can attenuate the rapid decline in vitamin D status, which is a key factor in the overall health of these patients. Vitamin D therapy for other diseases may also benefit from coadministration of CYP24 inhibitors.
Finally, the use of dual-action vitamin D compounds that simultaneously act as potent agonists of VDR transcriptional activity and as potent inhibitors of CYP24 activity, may serve as self-potentiating therapies that prove superior to the single action compounds currently serving as therapies of first choice.
Christian Helvig, Ph.D., is research director at Cytochroma, and Martin Petkovich, Ph.D. (martin.petkovich@ cytochroma.com), is CSO at Cytochroma and professor of biochemistry and pathology at Queen’s University. Web: www.cytochroma.com.