June 15, 2010 (Vol. 30, No. 12)
Getting the Most Out of Cells for Research, Manufacturing, and Gene Therapy Use
Improving the productivity of production cells, a major goal of biomanufacturers, involves the sequential application of cell-line engineering and optimization of media, feed, and process. Experts agree that this sometimes iterative process, like the development of higher human attributes, depends on both nature and nurture.
Crucell’s PER.C6® cell line illustrates the necessary balance between these two factors. The volumetric productivity of PER.C6 has become legendary, with the current titer record of 27 g/L at harvest and more than 40 g/L if you subtract the cell volume and count only the supernatant.
The success of PER.C6 is based on the cell itself, a human retina-derived cell that provides human-like glycosylation and all attendant benefits. “Processors don’t have to worry about undesirable post-translational modifications with PER.C6,” observes Kathryn Golden, a scientist in upstream processing at Percivia, a joint venture between Crucell and DSM that specializes in bioprocess applications of PER.C6.
But the role of post-cell-engineering optimization cannot be overstated either. Percivia’s mandate is to improve its parent company’s prolific cell line through media, feed, and process strategies. One such improvement is implementation of Refine Technology’s alternating tangential flow (ATF) technique, which perfuses fresh media through the culture while removing byproducts. ATF reportedly permits PER.C6 cells to grow to extremely high concentrations, up to 150 million cells/mL, in less than two weeks. Data shows that ATF generates viable biomass at ten times higher concentration than conventional cell culture.

Percivia is engaged in fundamental and applied research on the use of PER.C6® cells for the mass production of recombinant therapeutic proteins.
Speed to Process Development
With its licensing of zinc finger nuclease (ZFN) technology from Sangamo, SAFC Biosciences gained the capability to knock genes in or out with high specificity. According to Bruce Lehr, director of development, ZFN generates clones within six months. SAFC works directly on customers’ cells or provides them with the ZFN kits to do the work themselves. SAFC expects to offer a full-spectrum service that combines cell-line engineering services with optimization based on media, feed, and processes within three years.
ZFNs are artificial restriction enzymes in which a zinc-dependent DNA-binding protein and a DNA-cleaving enzyme are fused, thus combining highly selective recognition with DNA splicing. ZFNs have received a lot of attention from medical researchers, who use the enzymes to knock out genes.
ZFNs work by removing a gene sequence, leaving behind a double-stranded break in the DNA backbone. In the absence of a suitable template the strands will often close perfectly, resulting in a clean deletion. When a homologous template strand is available it will insert into the break.
ZFN technology has been used to create knockout rats. In April 2010, a group from Singapore used the methodology to generate a zebrafish model for Parkinson disease. And recently, the Michael J. Fox Foundation awarded a one-year, $232,000 grant to Sigma Advanced Genetic Engineering (SAGE) Labs to develop a more convenient Parkinson model in rats. Researchers at SAGE are expected to use the company’s CompoZr ZFN technology to create the model.
Knockout/knockdown strategies can have a tremendous impact on cell-line optimization for biomanufacturing, an area in which SAFC has been active. Lehr notes the potential for inactivating genes that code for apoptosis, host-cell proteins, or critical post-translational modifications such as glycosylation, methylation, or phosphorylation. One SAFC customer asked the company to delete a marker on the production cell that was homologous to the target receptor in humans.
SAFC has thus far focused on CHO cells, the workhorses of monoclonal antibody manufacturing. As it works on these production cells, the company is simultaneously matching media and feeds, to “optimize productivity from a very early stage,” according to Lehr. The goal is to introduce platform production technologies that will assist small biotech companies and contract manufacturers to develop cells and move them into production rapidly.
“Large companies are already using platform technologies but the next tier down and many CMOs lack the time or expertise to develop them,” Lehr reports. “Having platform manufacturing methods will help these companies attract customers.” Another boost to platform methods, he says, will be the eventual approval of biosimilars.
Not Just for Manufacturing
The value of cell-line optimization is not limited to biomanufacturing. For example Cellectis is involved in improving cells for research and gene therapy as well as production. The company’s lead technology is based on meganucleases, or “DNA scissors” that selectively remove genes. Meganucleases have been used for at least 25 years, but Cellectis claims to have engineered these enzymes for greater specificity.
Meganucleases, which are found primarily in single-celled organisms, algae, and some plant organelles, cut chromosomes at specific locations. Despite their relative rarity in nature, meganucleases may be inserted into numerous cell types, where they dependably “cut-and-paste” at target sequences provided the genome contains a specific, 12–30 base pair target sequence. Long recognition sequences are the key to the enzymes’ specificity. If the sequence does not exist naturally it must first be introduced, which has been the major limitation of natural meganucleases.
Cellectis has discovered how to adapt these enzymes to a much broader range of DNA sequences by modifying the meganuclease recognition capability and selecting specific enzymes through high-throughput screening.
The company has thus far worked on workhorse mammalian cells such as HEK293 (human), NIH333 (mouse), and CHO-k1 (hamster), which together cover major areas of interest to both researchers and biomanufacturers. “Clients use our products mostly for drug screening, protein production, and functional genomics,” explains Marc Le Bozec, CEO. Cellectis has enjoyed a “100 percent success rate,” according to Le Bozec, in deleting and inserting genes to produce stable clones.
Cellectis sells kits known as meganuclease recombination systems consisting of specific meganucleases paired with a target DNA template sequence. Users, says Le Bozec, have been 80% successful in targeting specific sites on the genome for gene deletion, insertion, or replacement.
“Each kit provides ten experiments, of which eight should produce a stable, desired clone within four weeks. Moreover it will generate a single copy of a single gene precisely where the customer desires it.” This provides the opportunity, for example, to quantify the activity of two genes located in the same genetic environment. “You can now compare apples to apples,” Le Bozec explains.
By the end of the year the company hopes to introduce kits for knocking down genes by insertion of siRNA. It is planning a next-generation product in 2011 for gene knockout.
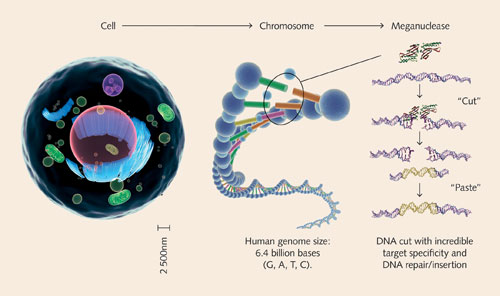
Cellectis specializes in the development and production of meganucleases (DNA scissors) for in vivo genome surgery. The company says that its products are programmed to induce unique, site-directed, double-strand DNA breaks in a living cell and can be used for a wide range of applications. [Valentina Herrmann]
Combination of Factors
Rapid gene-splicing techniques notwithstanding, cell-line development takes time in the real world and involves much more than simply introducing genes and firing up a bioreactor. For example Xoma has adapted CHO-k1 cells, a standard production line, to suspension growth and animal component-free media, which “allows more rapid transfer from cell development into process development,” according to Arnie Horwitz, Ph.D., senior director for expression technologies.
During cell-line development Xoma relies on proprietary expression vectors that achieve high expression of the desired gene without conventional gene amplification. Dr. Horwitz calls these “multigenic” vectors because they each contain more than one copy of the desired gene. “This approach increases gene copy number in stable cell lines while eliminating the need for gene amplification.”
Xoma’s cell-line work is complimented by media and feed development efforts that begin with a platform process. The goal is to maintain cells at high density for 14 days or longer to maximize productivity while maintaining product quality. Glycosylation and structural changes are watched closely through “sophisticated analytical tools” that have become part of Xoma’s cell-line selection and optimization process.
Success, Dr. Horwitz says, depends on close interaction between development groups upstream and downstream from cell-line development. “This occurs easily at Xoma because we’re small and able to maintain fluid communication between groups.”
Once cells with the right attributes emerge, they are transferred to separate groups that evaluate them in bioreactors. Quality assessment comes into play here as well, since higher-producing cells may not be the ones that produce product with desirable quality attributes.
“The question of assessing value to cell-line and process development comes up quite often in meetings,” Dr. Horwitz notes. “Both functions are critical. Without transfection, without stable cells possessing acceptable specific productivity you have nothing—everything you do downstream of that becomes more difficult.”
The nature vs. nurture conundrum in cells parallels the evolution of biomanufacturing itself. Biotech was first interested in expression vectors, but for the last 15 years investigators have become more concerned with process optimization. Today, Dr. Horwitz says, the focus is on arriving at a suitable cell line quickly. Hence the interest in rapid transfection and high-throughput clone-selection techniques such as ClonePix FL from Genetix, which claims to reduce selection time by more than 60%.
Sidebar: Not Just for Recombinant Proteins
The more that is learned about cell genetics, the greater the ability to manipulate all sorts of production cells. Researchers from the University at Buffalo have discovered the most efficient production system yet for isoflavonoids, multiring organic molecules of great interest in pharmaceuticals.
Mattheos Koffas, Ph.D., associate professor in the department of chemical and biological engineering, has engineered brewer’s yeast to produce isoflavonoids at titers of 50 mg/L. These quantities at first seem meager in light of PER.C6 productivity of 40 grams per liter, but Dr. Koffas notes that early fermentations for polyketide antibiotic manufacturing yielded only about 1 mg/L of product.
The technique opens up new avenues to the synthesis of thousands of natural and non-natural isoflavonoids, says Dr. Koffas, whose group has optimized the yeast for the combined action of three isoflavonoid-synthesizing enzymes using common flavanone precursors. Several compounds have already shown activity in estrogen receptor binding, an attribute of several marketed breast cancer therapies.
The required enzymes—P450 monooxygenases, p45 reductase, and a dehydrogenase—are normally found in plants, where they naturally produce isoflavonoids in excruciatingly low concentrations. Screened enzymes were inserted into the yeast, and the organisms were selected for optimal productivity. The organisms are versatile, producing a wide variety of products depending on the starting flavanone supplied.
Creating cell- (vs. egg-) derived influenza vaccines has focused on several cell types that grow flu virus effectively. Viruses are particularly fond of one such line, Madin Darby canine kidney (MDCK) cells, but MDCK cells are stubbornly attachment-dependent, which hampers large-scale viral vaccine production and harvesting.
A group at the National Institute of Diabetes and Digestive and Kidney Diseases at NIH led by Joseph Schloach, Ph.D., has identified a human gene, siat7e, that disrupts attachment of MDCK cells. Expressing the gene in anchorage-dependent MDCK cells tranforms the cells rapidly into suspension cells.
Traditionally, the transformation is achieved in MDCK, CHO, NS0, and other cell lines through serial passage, which is slow. Dr. Schloach’s technique achieves suspension cells at once, at concentrations of just under 1 million cells/mL. MDCK cells that express siat7e not only produce vaccine that is identical to virus that infects attachment-dependent cells, but generate the critical HA1 (hemagglutin-1) antigen at levels 20-fold higher than the parent cells, he says.
Can this technique be applied to short-circuit attachment-dependence of other cell types? “It is possible, but you can never be sure of the results since the transformation is a complex process and depends on several factors,” Dr. Schloach adds.



