February 1, 2006 (Vol. 26, No. 3)
Lack of Screening Assays Is Slowing Development of Selective Kinase Inhibitors
The central role of protein kinases in signal transduction and recent clinical success with small molecule kinase inhibitor drugs, most notably Gleevec (Novartis), have generated intense interest in kinase enzymes as therapeutic targets.1 Next to G-protein coupled receptors, protein kinases have become the most intensively screened target class.2
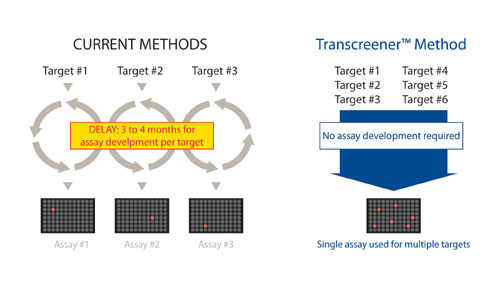
However, the ability to validate and pursue new kinases for drug discovery is being hampered by a lack of screening assays capable of accommodating diverse kinase isoforms and their corresponding acceptor substrates. Most kinase assay methods rely on detection of a tagged phosphopeptide, and significant development or optimization is required to accommodate each new peptide sequence recognized by a particular kinase.
Though there is overlap in substrate specificity among related kinases, there is no consensus sequence that is phosphorylated by a large number of kinases.3 The time and money required to develop assays for kinases with novel recognition sequences is slowing the identification of selective inhibitors for validating new kinase targets or as potential lead molecules (Figure 1).
Moreover, new therapeutic strategies are resulting in increased demand for kinase screening assays that can accommodate nonpeptide acceptors. Examples include:
Native proteins
Non protein substrates (lipid kinases)
Autophosphorylation
The assay development bottleneck extends further downstream in the discovery process as well.
At some point following a primary screen, secondary screens are often run against a panel of 10100 kinases to obtain selectivity profiles for the most promising hits. These profiling studies generally include potency determinations for compounds where significant off-target activity is observed. The available nonradioactive HTS assay methods are not suitable for quantitative assays with a broad spectrum or kinases, and studies have shown that there are significant differences in the identity and potency of hits determined with different HTS assays methods.4
For these reasons, the bulk of selectivity profiling is done using the traditional 33P-radioassays. Most pharmas contract this work, at considerable expense and inconvenience, to service providers who are willing to assume the regulatory and disposal requirements.
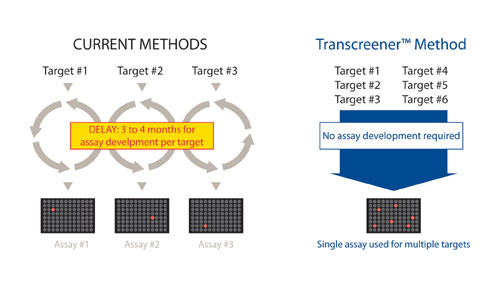
The Transcreener KINASE Assay overcomes the assay development bottleneck, enabling rapid incorporatin of diverse kinases with their corresponding acceptor substrates into HTS.
Flexibility in Kinase Screening
The Transcreener KINASE Assay from Bellbrook Labs (www.bellbrooklabs.com) is a universal HTS assay that relies on immunodetection of ADP, the invariant reaction product of all kinase reactions (Figure 2). This enables screening of any kinase enzyme, with any acceptor substrate, using the same assay and reagents. It is a homogenous or mix-and-read assay method that uses fluorescence polarization as a detection method.
The enzymatically generated ADP displaces a fluorescent tracer from an ADP antibody, resulting in decreased fluorescence polarization. Fluorescence polarization is a broadly accepted detection mode for HTS; it is used extensively for kinases5-7 and a number of other enzymatic and ligand binding reactions.7,8
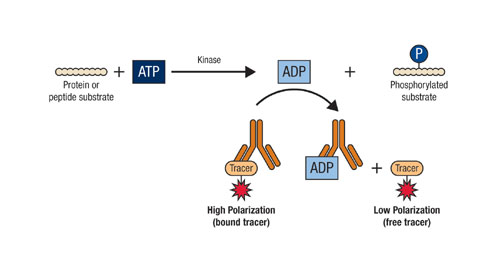
The Transcreener KINASE assay is based on detection of ADP by fluorescence polarization immunoassay. ADP producted in the kinase reaction displaces a fluorescent tracer from antibody, resulting in a decrease in its fluorescence polarization.
The key reagents for the Transcreener KINASE Assay are an antibody specific for ADP and a fluorescent conjugate to the donor productcalled a tracerthat retains its antibody-binding properties. The major technical hurdle was the development of antibodies that bind the ADP with minimal crossreactivity with ATP.
In addition to the structural similarity of the di- and triphosphates, the challenges include the small size and lability of the antigens. As shown in Figure 3, monoclonal antibodies have been generated with nanomolar affinities for tracer and 100-fold selectivity for ADP over ATP.
Transcreener Assays have also been developed for glycosyltransferases (UDP detection), and additional assays are under development for sulfotransferases, methyltransferases, and other families of group transfer enzymes.
The total time required for development of Transcreener detection reagents ranges from four to eight months, depending on whether a polyclonal or monocolonal antibody is used. Once the reagents are available for a family of group transfer enzymes, no further assay development is required other than buffer optimization for specific enzyme isoforms or adjustment of antibody concentration to tune the assay sensitivity.
This stands in contrast to most of the other group transfer assay methods, where new detection reagents, such as antibodies or fluorescent probes, must be developed for individual enzymes or subgroups of enzymes within a family.
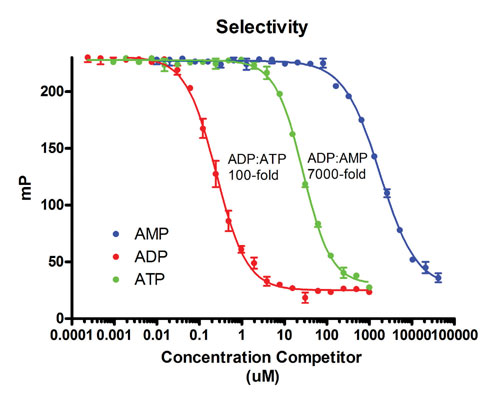
Fluorescence polarization competition curve for AMP, ADP, and ATP with antobody and tracer developed for Transcreener Kinase Assay.
The flexibility of the Transcreener HTS Platform is illustrated by the ability to detect four different protein kinases were detected, each with a different acceptor substrate, using the same detection reagents (Figure 4), demonstrating the flexibility of the Transcreener HTS assay platform. Note that one of the acceptors used was a protein, Histone H1, instead of the more typical peptide. The ability to use native protein acceptor substrates is critical for kinases, such as Raf-1, where no peptide substrates have been identified.
In addition, investigators have recently initiated efforts to develop kinase inhibitor drugs that bind at allosteric sites. It would be optimal to determine the binding properties and effects of such an allosteric inhibitor using a native protein acceptor; different effects might be observed with a nonphysiological peptide substrate.
Transcreener is a nonradioactive, generic kinase assay platform with the flexibility to accommodate any protein kinase and any acceptor substrate. The availability of such a platform will simplify selectivity profiling and accelerate the screening of difficult kinases that require native protein substrates.
Moreover, by providing a single method and data output for all primary and secondary kinase screening functions the Transcreener platform has the potential to improve the overall kinase drug development process. Use of the same assay method for primary screening and selectivity profiling will enable a more integrated and iterative approach for the development of highly selective inhibitors, leading to more efficacious drugs with fewer side effects.
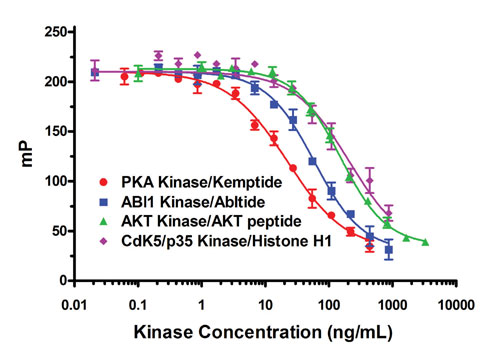
A single set of reagents can be used to assay any kinase using peptide or protein substrates.