March 1, 2012 (Vol. 32, No. 5)
There are two functionally different types of DNA transfection (with a gray area between). On the one hand, stable transfections allow newly introduced genetic material to be passed down as part of the fixed genome. On the other hand, transiently transfected cells carry transgenes episomally.
We rarely hear much about transfection—it’s more of a behind-the-scenes player. Yet whether and why to make stable lines vs. transiently transfect, as well as seeming nuances such as which host cell line to use, how to introduce the DNA, under what conditions to culture the cells—both short term and long, and even what kind of media to use, can lead to (or avoid) untold frustrations in the process of generating the cells for manufacturing or experimental purposes.
Such nuances were certainly on the mind of researchers who shared their transfection experiences at Informa’s “Cell Line Development and Engineering” meeting held last month.
Science doesn’t always proceed by “aha!” moments—sometimes it’s incremental advances over a myriad of seemingly small parameters cumulatively coming together that lead to big improvements. That is the case at UCB Pharma UK, where Bernie Sweeney, Ph.D., group leader for protein expression and purification, generates antibodies for early-stage research and preclinical development.
“Back in 2006, we were making about five grams over the year,” she said, comparing that to the 40–50 grams of protein they’re producing now from an equivalent number of transfections.
Over the past five years UCB has engineered a CHO host overexpressing both XBP-1 and ERO1-Lα, two key regulators of protein secretion and cellular oxidative stress, respectively. Use of this host has resulted in routines titers between 400–700 mg/L.
Supplying reagents for several ongoing projects at early-stage discovery through in vivo studies means that their system needs to be able to cope with both large- and small-scale production. At the moment its preferred method of transfection is electroporation using individual cuvettes, which for large-scale production requires “an awful lot of cuvettes,” Dr. Sweeney remarked.
Her group recently delivered a 13 g batch of purified IgG1 in 10 weeks, which would have taken 16–20 weeks using a bulk pool or stable cell-line process, she noted. While labor-intensive, the great benefit of using a transient expression system to generate gram-quantities is its ability to reduce timelines.
Transient transfection is generally seen as a short-term solution for making small amounts of a protein. Yet as several speakers argued, there are distinct advantages that transient transfection offers over its more laborious stable cousin.
Among the most obvious is the time it takes to generate a stable cell line—a major barrier for discovery and preclinical programs that require the screening of multiple different components in conjunction with one another.
And this is especially the case when those components are parts of a complex multimeric protein. Manuel Carrondo, Ph.D., professor of chemical and biochemical engineering at, and CEO of, Portugal’s Instituto de Biologia Experimental e Tecnológica (iBET) and his team developed a HEK293-based virus-producing packaging cell line that allowed for easy replacement of the gene(s) of interest by homologous recombination.
Once the high-producing line was established, it could be reused to generate transient lines with predictable high viral production without the need for further exhaustive clone screening. Compatible retroviral vectors could be swapped in and out in a matter of weeks, in a process called recombinase mediated cassette exchange (RCME).
Typically recombinant viruses and virus-like particles (VLPs) would be produced by co-infection. Jumping from the co-infection strategy to targeting where we know that there is a single insertion and where it is, allows for better management of both the stoichiometry of the unit and also the timing, Dr. Carrondo noted.
“For example, you need a given protein to arrive at a later stage to cover up or to link to the one that has to be there at an earlier stage. Those are thermodynamic and stoichiometric issues involved. By doing this with our recombinant mediated cassette exchange, you can test many more options for VLPs or multimeric proteins in a shorter time than through co-infection and screening.”
Production and assembly for the different multimeric proteins can change much faster, vastly improving the iterative process at the preclinical stage, he said.
E. coli and yeast are surely simpler systems, but animal cells are needed to produce complex proteins with post-translational modifications. Dr. Carrondo’s group recently set its sights on using this “tag and target” methodology for VLPs and multimeric proteins in insect cell lines.
Compared to mammalian cells they are generally more robust, metabolically easier to deal with, less expensive to maintain, and are also more genetically stable.
Oh HEK
Be that as it may, CHO cells are still the dominant stable producer lines in the pharmaceutical industry. Yet because R&D material is principally generated in HEK293-based cells, so as to minimize the (real and perceived) post-translational modification dissimilarities between transiently and stably expressed genes, there has been a call to develop a viable large-scale CHO transfection platform as an alternative. Regulatory submissions, too, would be simplified by using a common host to generate both preclinical and clinical matter.
Yves Durocher, Ph.D., research officer at the National Research Council Canada, has been heeding the call. His lab has developed a simple and robust transfection process that allows CHO cells to compete with the ease of use and expression levels of 293 cells.
The automation-compatible process utilizes serum-free medium (facilitating the recovery of secreted proteins), does not require medium exchange prior to or following transfection, and makes use of suspension cells, allowing for scalability.
“We are basically using the same approach, same protocols, and the same vectors to transfect CHO cells,” he said. As such they typically express every new target in both 293 cells and CHO cells, and test lines in parallel for the best expression and product quality/activity.
In some cases there have been differences in glycans or other post-translational modifications between the two hosts, which can be critical for many biological functions. This can be the result of rodents expressing different enzymes and in different proportions than humans, or by the fact that ovary metabolism may differ from that of the kidney.
“The more hosts we have, I think, the better,” commented Dr. Durocher. “It provides you with other options for different targets.”
But all else being equal, Dr. Durocher thinks that companies that already have stable CHO expression platforms for manufacturing biologics would rather use a large-scale CHO transient transfection system than use different platforms for research and production.
For most of the small percentage of proteins that can’t be made in the cells as they are now, “you can still stay with CHO cells and just engineer them to provide better biologics,” he said. “But of course you also have the opportunity to switch hosts and start from scratch in developing a new platform.”

The flask on the left is a control CHO culture (mock-transfected) while the two on the right are CHO cultures transfected with a plasmid expressing a secreted mRFP1 (monomeric red fluorescent protein) fusion protein. The expression level reached here is slightly over 300 mg/L. [National Research Council Canada]
Transfection Is Infection with DNA
Of course, there are certain circumstances when CHO cells just won’t do—like, for example, for the making of enveloped VLP vaccines. “Intrinsically you can’t perform the viral clearance that’s required from products in rodent cell lines,” explained Richard Schwartz, Ph.D., chief of the vaccine production program lab at the NIAID’s Vaccine Research Center.
“If you make a protein or product in CHO, you are required to show about 13 logs of viral clearance in your production process. So you generally have to do low pH viral or detergent inactivation and there’s a viral filtration.
“Low pH will basically destroy the VLP immunogenicity—it completely changes the envelope structure and it would destroy the actual activity of the VLP. And you can’t do a viral filtration, because VLPs are essentially the size of a virus and so would be removed by filtration.”
Add to this that enveloped VLPs would literally kill the host cells as they are produced by carrying the lipid membrane to form the VLP, and “this lipid membrane damage causes greatly decreased cell growth if not cell death, so you’ll never be able to passage the stably transfected cells enough to make master cell banks or to expand them into your final bioreactor,” he said. So what to do?
Dr. Schwartz has been using a transient HEK293 expression platform for early-phase clinical trials of a nonreplicating VLP vaccine for Chikungunya, a virus endemic to parts of Africa and Asia that can cause debilitating symptoms. The host cells can be expanded prior to transfection. “I don’t care about the cells at my last step of the process,” Dr. Schwartz confessed. “All I care about is the product.”
Transient transfections are notoriously difficult to scale up. Commercial media is highly inhibitory to these transfections, and so you have to do a large-scale centrifugation or media exchange of some sort—which becomes a problem at multiliter quantities.
Dr. Schwartz’ lab is currently scaling up production into 50 liter disposable bioreactors, as well as looking at inducible promoters, fed-batch processes, and other improvements for the next generation of VLP production.
Fortunately, the dosages of viral vaccines are so much smaller than those of therapeutic monoclonal antibodies—in the range of 20–100 micrograms, versus perhaps grams of mAb. So even with the productivity they’re currently achieving—about 100 mg/liter in 1 liter flasks—it’s easy to make sufficient doses for early-phase trials.
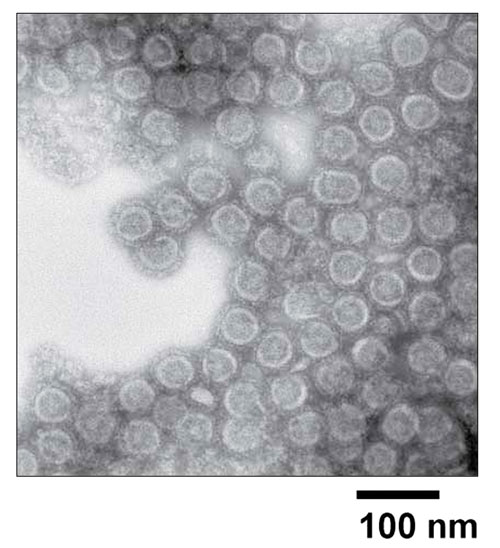
NIAID Vaccine Research Center scientists have developed a new vaccine that protects against CHIKV infection of nonhuman primates. They have found that selective expression of viral structural proteins gives rise to virus-like particles (VLPs) in vitro that resemble replication-competent alphaviruses. Immunization with these VLPs elicited neutralizing antibodies against envelope proteins from alternative CHIKV strains.



