December 1, 2009 (Vol. 29, No. 21)
Systems-Level Technology Has Found Niche in Biofuel Production for Now
Craig Venter, Ph.D., of human genome fame and the Craig Venter Institute, created a stir with his August publication in Science of a technique for transferring a wholly engineered genome into a bacterium. While it does not create artificial life (which Dr. Venter claims will be forthcoming), this work justifies Dr. Venter’s claim that, “We’re moving from reading the genetic code to writing it.”
While de novo creation of new organisms remains a goal for biologists, every success in biotechnology has involved more mundane engineering of well-characterized systems and organisms—the work-horses of biotech. This was illustrated by the first two great waves of biotechnology—genetically modified foods and therapeutic biotech, through which “ordinary” cells or organisms receive the instructions to produce recombinant products. On that basis, one could argue that all biotechnology is “synthetic” at some level.
But in the current jargon, synthetic biotechnology goes deeper. “It is about systems,” says A. Malcolm Campbell, Ph.D., professor of biology at Davidson College, “not manipulating one or two genes, but whole pathways.” For example, producing a compound in a microbe that does not normally make it might involve splicing in some genes and deleting or turning off other genes.
Dr. Campbell achieved recognition in 2008 for solving the “burnt pancake” problem with bacteria. The problem involved calculating the minimum number of manipulations required to stack some number of pancakes burnt on one side, from largest to smallest, with each burnt side oriented the same way. Instead of pancakes, Dr. Campbell used an E. coli plasmid, which, when arranged in a particular order, confers antibiotic resistance.
Dr. Campbell introduced a plasmid containing all the components for antimicrobial resistance, but in scrambled order. Using the Hin invertase enzyme as his “spatula,” Dr. Campbell turned the enzyme on and off, and noted how many “flips” were required to confer resistance. At the end of three flips a few cells survived, indicating that resistance had been achieved. “We arrive at the solution by detecting a new phenotype,” Dr. Campbell says.
One of Dr. Venter’s goals, announced in 2007, was the creation of a synthetic bacterium that produced high-energy liquid fuels from waste carbon (including plastic) feedstock. An online search of synthetic biotech will uncover many similar projects.
Co-founded in 2001 by University of California, Berkeley, bioengineer Jay Keasling, Ph.D., Amyris Biotechnologies uses bioengineered yeast to produce high-performance liquid fuels and pharmaceutical intermediates.
The company’s first project involved yeast-produced artemisinin, a malaria treatment in great demand in the developing world. Developers have improved yield for a key intermediate in the production of artemisinin by a factor of 1 million in just two years.
Gevo uses E. coli to produce butanol through a process it licensed from James Liao, Ph.D., who heads the metabolic engineering and systems biology laboratory at the University of California, Los Angeles. Butanol holds 50% more energy than ethanol, and since it does not mix with water, it can be transported through existing fuel pipelines. The molecular engineering involves diverting keto acids from E. coli into a metabolic pathway that produces four-carbon alcohols that are foreign to E. coli. The gene cassette responsible for this chemical diversion may be transferred to other organisms that may be further engineered to withstand high butanol concentrations.
Joule Biotechnologies recently unveiled Helioculture™, a process that uses microorganisms to convert sunlight and carbon dioxide into ethanol or diesel fuel. Unlike algae and other biomass-based fuel production strategies, Helio-culture does not generate biomass, requires no agricultural feedstock, uses unpurified brackish water, and operates on any terrain including nonarable land, the company says. The process also reportedly produces fuels directly, with little or no post-production processing.
Helioculture far outproduces production methods based on distilling or fermenting crops, according to the company. Joule estimates that it can manufacture 20,000 gallons of ethanol or hydrocarbon fuel per acre—a factor of between eight and ten times higher than corn-derived ethanol or distillation from cellulosic feedstock.
The company uses genetically engineered photosynthetic microorganisms to generate organic fuels that are collected by sweeping them, in gaseous form, from the headspace above their culture medium. The identity of the photosynthetic microorganism and its extensive genetic modifications are, for now, a trade secret.
Organics are purified by centrifugation or distillation. The vessels, dubbed SolarConverters™, are modular, fully scalable, and resemble solar panels more than bioreactors. The microorganisms take three days to grow from seed cultures and work for up to eight weeks. Because it uses waste CO2 generated from industrial processes and energy production, Helioculture is carbon-negative, says CEO Bill Sims.
“What differentiates Helioculture is that it does not involve biomass, but the direct conversion of sunlight into fuel. Other technologies have attempted to do this, unsuccessfully, using photobioreactors.”
Sims believes Helioculture is close to being cost-competitive with oil and plant sources of ethanol and hydrocarbon fuels. Target prices for those two fuels are $80 and $50 per barrel equivalent, respectively.
University of California, San Francisco school of pharmacy professor Christopher Voigt, Ph.D., uses an entirely different approach—building up hydrocarbons from methyl halides derived from biomass and salt. The gasoline derived from Dr. Voigt’s process is indistinguishable from petroleum-derived gasoline and would not require new vehicle engines, he says. The biotransformation uses cellulose-rich, nonfood crop waste or grasses and would therefore not displace resources used to produce food.
The process involves a symbiotic relationship between a bacterium and baker’s yeast (S. cerevisiae). The bacterium, originally isolated from a French garbage dump, process agricultural waste and converts it to acetate, which the yeast transform into methyl halides. Methyl halides have been previously explored as intermediates for converting natural gas to gasoline, but this is the first instance where the compounds were obtained from waste biomass. The second build-up step uses zeolite (volcanic rock) catalysts to polymerize the single-carbon units into longer-chain hydrocarbons, for example, the fuel components of gasoline.
Codexis has carved out a niche in biofuels, pharmaceutical intermediates, and industrial biocatalysis. Through classical molecular techniques, the company adapts enzymes to chemical processes—not the other way around, as is more common. Not everyone would agree that Codexis’ technology falls cleanly into the category of synthetic biotechnology or synthetic biology. Purists limit the designation to new or synthetic genomes, biomimetic systems, or entirely new life forms, but this definition is perhaps too limiting.
The University of California, Berkeley, in describing a biofuels project, defined synthetic biology as: “the design and construction of new biological entities such as enzymes, genetic circuits, and cells, or the redesign of existing biological systems. The field builds upon advances in molecular, cell, and systems biology and seeks to transform biology in the same way that synthesis transformed chemistry and that integrated circuit design transformed computing.”
Lori Giver, Ph.D., who heads systems biology at Codexis, admits that the definitions are still fuzzy. “Every application of directed evolution to a protein or organism involves making novel genes or pathways, but I’m not sure how well the definition [of synthetic biology] fits.”
Codexis develops novel biocatalysts from enzymes which, in their native state, often bear little resemblance to the final catalyst product. Natural enzymes work in a mostly aqueous milieu, whereas industrial enzymes must operate under less ideal conditions. Using DNA shuffling, the enzyme is modified numerous times until it achieves the desired properties based on what Dr. Giver calls an optimized or ideal process. “That process dictates how good the enzyme has to be.”
Matching enzymes to industrial processes is actually more complex than that and involves some give-and-take. When asked if she could design enzymes that work in high-boiling solvents, Dr. Giver notes that the purpose of using enzymes is specifically to avoid conventional, high-energy, solvent-intensive processes. “You don’t want an ideal process to use refluxing DMSO, you want something greener that does not require as much heat or solvent.”
Codexis has designed a biocatalyst cocktail that synthesizes the chiral side chain of atorvastatin, the active ingredient in Lipitor. The new process reduces the volumetric productivity of two key reactions 100-fold and 4,000-fold, while reducing cost of goods by up to 70% and capital expenditures by 35%.
Semantic sticklers will note that biocatalysts have been around for a while, and lack the “systems” component of synthetic biotechnology. In reality, biocatalysis is classic systems biology, which apparently qualifies. The systems-level engineering that created the atorvastatin synthesis was extensive, led to new reactions, and fit the classic definition of synthetic biology, according to Dr. Giver.
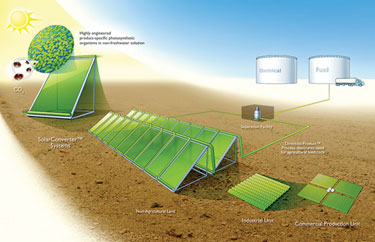
According to Joule Biotechnologies, its Helioculture™ technology uses sunlight to convert CO2 directly into Joule™ liquid energy.
Roadblocks
Numerous hurdles will confront synthetic biotechnology as the field expands. The creation of deadly super-bugs is an issue that has been raised since the first genetically engineered cells were created. We have avoided biological Armageddon up to now because the number and types of foreign genes inserted into cells and organisms has been carefully controlled—not by law, but through commercial expediency. The likelihood that such problems will arise in the future increases as researchers focus more on systems vs. single genes. The possibility becomes more real for completely artificial life, although Dr. Venter might disagree.
For the time being, synthetic biology faces more practical issues related to the constant evolution of cells and microorganisms. “It’s impossible to overlook this, to trust engineered living systems not to change over time,” says Dr. Campbell. “The big difference between bioengineering and electrical or mechanical engineering is organisms that undergo selection pressures, that continue to evolve even though that evolution is not part of our plan.”
Campbell cited a study that found that 10% of the time, knockout yeast strains spontaneously undergo gene deletions and duplications that were not designed into the organisms. Another paper found that the live BGC tuberculosis vaccine had been changing unnoticed since the 1960s. If simple organisms can evolve with no apparent evolutionary pressures, systems-wide manipulations may be prone to even greater diversity over time. The potential implications for cells and organisms used in biomanufacturing, particularly over many years, are worth considering.



