May 1, 2017 (Vol. 37, No. 9)
Thorsten Peuker Ph.D. Vice President of Design & Implementation Sartorius Stedim Biotech
Bioprocessors See Opportunities in Flexible and Modular Biomanufacturing Facilities
The biopharmaceutical industry remains in a state of permanent change. The requirements of patients as well as healthcare systems of different countries are leading to volatility in the demand for drugs. Hence, the question often gets asked: How can we overcome today’s challenges in biologics manufacturing? New processes and innovative technologies are enablers for more efficient and cost-effective drug development and manufacturing.
One of the most popular discussion topics over the past few years has been the “facility of the future.” The buzz phrase prompts important questions. Are there strategies we can employ to cope with the changes in the industry? Is there a single solution that will allow us to be ready for all our future requirements?
Chew et al. have discussed this in detail. They concluded that there are many initiatives ongoing but these have yet to be implemented holistically, particularly in GMP manufacturing.1 There is an urgent need for regulators and the industry to accelerate the adoption of new technologies, new methods of process and quality control, as well as other approaches that promote flexibility, lower costs, and higher quality.
In this article, we want to focus on single-use technologies and their effect on the design of facilities, highlighting modular approaches.
Requirements of the Industry
In many drug manufacturing companies, there are currently initiatives such as “factory of the future” or “manufacturing on demand” with the goal of identifying and describing the needs of the next generation of biological drug manufacture. Some industry organizations, such as the ISPE, have working groups are investigating these needs.1 The primary goals are to simplify the production process, make the process stable and reliable under current and future GMP requirements and, last but not least, to reduce waste.
New facilities will need to meet some basic requirements:2
- Apply single-use technologies for lower capital expenditures (CAPEX), from cell vial to drug product vial and from development to routine manufacturing
- Provide a platform for rapid transition between product development and commercialization
- Ensure rapid start-up and ease of compliance
- Be reusable and flexible as process and products change (New manufacturing strategies)
- Compliance and plant fit assessment, comparability of systems/transferability
- Development of automation and control strategies, including PAT, data analysis, real-time release
- Supply chain strategy/Security of supply (single-source vs. multiple sourcing)
The substantial growth of complex large molecules in the industry pipeline and advances in cell lines are driving new capacity demands for biopharmaceutical companies and their CDMOs. The attractiveness of marketed biologics, advances in manufacturing technologies, and increased productivity due to significantly higher titers have led to significant changes in capacity requirements. Innovative, highly efficient, flexible approaches and facilities to meet challenging capacity demands have become an imperative.
The types of capacity offered are also changing and are being based on new process platforms such as flexible, modular, and single-use technologies and continuous manufacturing. Demand for mammalian cell culture product-types have grown while antibody-drug conjugates (ADCs), vaccines, gene therapy, and cell therapy have also had an impact on capacity needs. There is a need to link the new process platforms with each new product category.
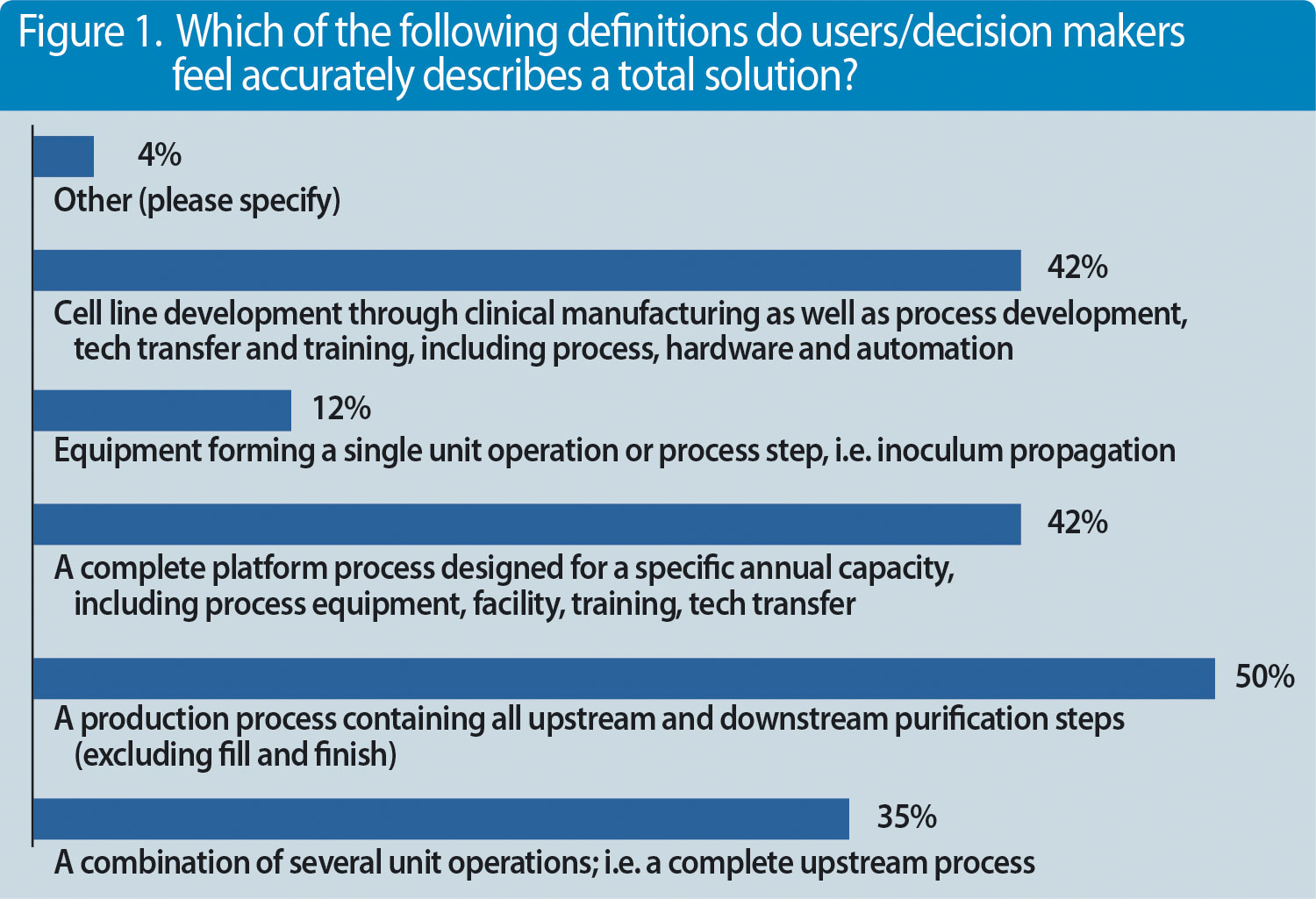
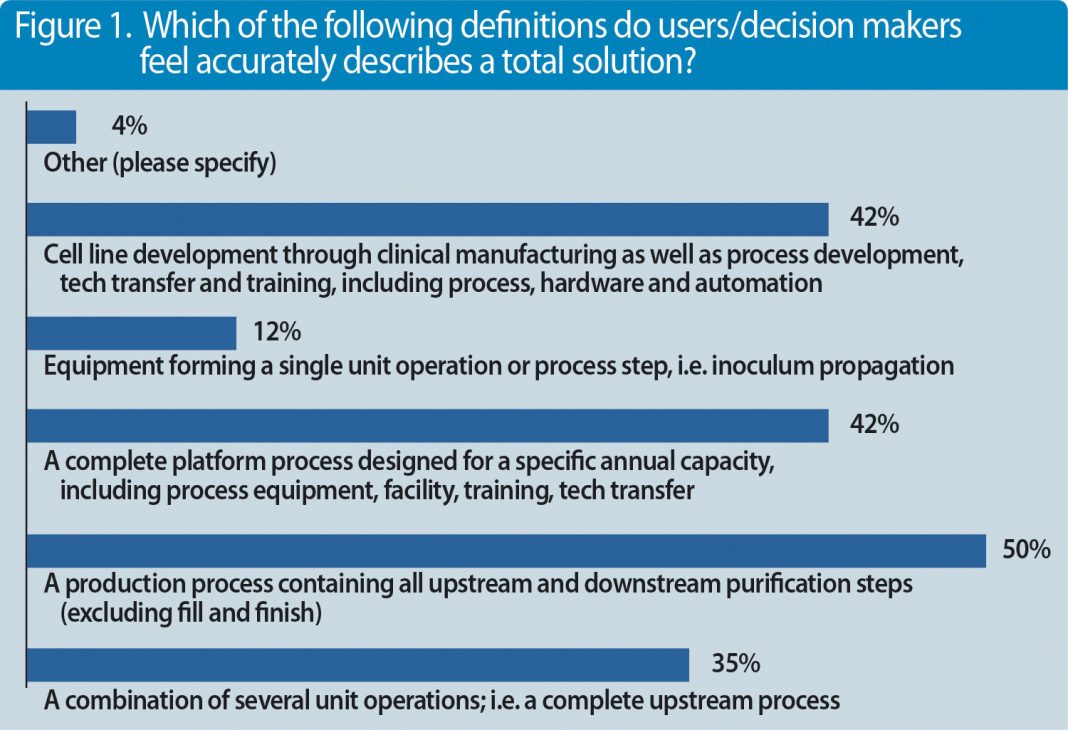
Figure 1. Which of the following definitions do users/decision makers feel accurately describes a total solution?
Process platforms for different applications (e.g., Sartorius Stedim Biotech’s Process4Success mAb, ADC or vaccine initiatives) are designed for flexible manufacturing operations. Consequently, rapid changeover to other processes is an important task that this initiative takes into consideration. The Process4Success approach intends to benefit not only CDMOs but also the industrialization departments of originators.
This initiative aims to reduce engineering efforts not only during planning and execution but also during equipment qualification. Sartorius has shown that complete 2,000 L single-use mAb greenfield plants for clinical and commercial production can be designed, executed, and tested within 18 months.3
But what else are decision makers and professionals looking for? We conducted a survey recently with almost 100 participants and asked the question, what represents a complete solution?(Figures 1 & 2).
Companies that are investing into new manufacturing capacities or want to refurbish existing capacities are looking for end-to-end integrated solutions. Integration may even include process development services such as cell-line development. There are different drivers for this need. First and foremost, new processes are pushing companies to invest into entire process platforms containing new (single-use) technologies. Lack of internal engineering resources or the need to reduce upfront investment costs forces corporations to work with solution providers like Sartorius that have competencies in these fields.
Once a partner has been selected, the implementation of flexible, modular facilities in a fast and efficient way is critical in order to reduce time to market.
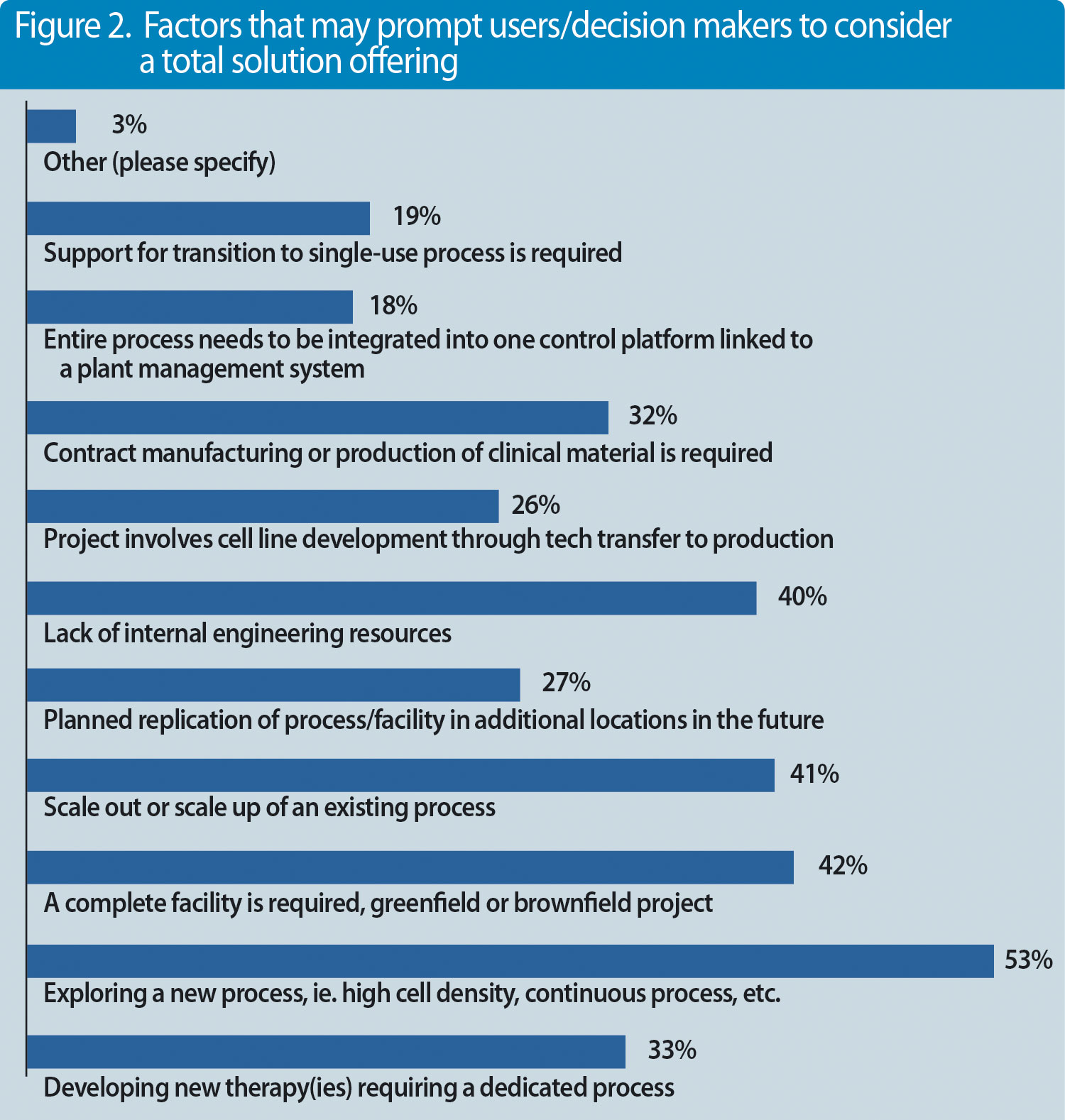
Figure 2. Factors that may prompt users/decision makers to consider a total solution offering
Solutions for the Industry
Flexible manufacturing environments and single-use systems have eliminated many of the classical constraints on biopharmaceutical processes and have enabled designers to engineer modular bioprocesses and house them easily in properly classified environments. The ability to repurpose, improve, change, and reuse these core elements of a facility impacts the industry from large pharma to small biotech companies.
Whether planning new facilities, establishing first-time manufacturing capabilities, or executing new supply chain strategies by decentralizing manufacturing, companies now have with modular, single-use technologies clear alternatives to fixed piped, stainless-steel equipment. The biopharmaceutical industry will approach the goal of implementing its future manufacturing strategies as suppliers continue to develop innovative products and collaborate effectively with their customers.
Working with single-use closed systems offers the opportunity to downsize considerably higher classification cleanroom areas. Large-volume support solutions can be stationed in a CNC (controlled nonclassified) environment and fed through the wall through a transfer hatch into the higher classification areas. Sartorius has many examples of optimized facility layouts offering a considerably reduced footprint (Figure 3).
Surfaces of ISO8 and ISO7 cleanrooms can be reduced by an average of 20% with a design based on a single-use process train. Cleanroom volumes can be reduced by a further 26% through being able to reduce the usual cleanroom ceiling heights typically required for stainless-steel bioreactors. Overall, on average, one can downsize HVAC systems by around 33% by reducing air supply requirements, although this does vary on a project-to-project basis.4
Irrespective of whether a production facility is a retrofit or greenfield project, the process and the workflows must always be defined in order to develop facility layout. Logistics for material handling, segregation of critical steps, and space requirements for work in progress as well as storage, plus provision of the utilities required for process and cleaning needs at every critical process step, must be fully specified. Finally, support spaces required and their interactions with the process path must be clearly defined.4
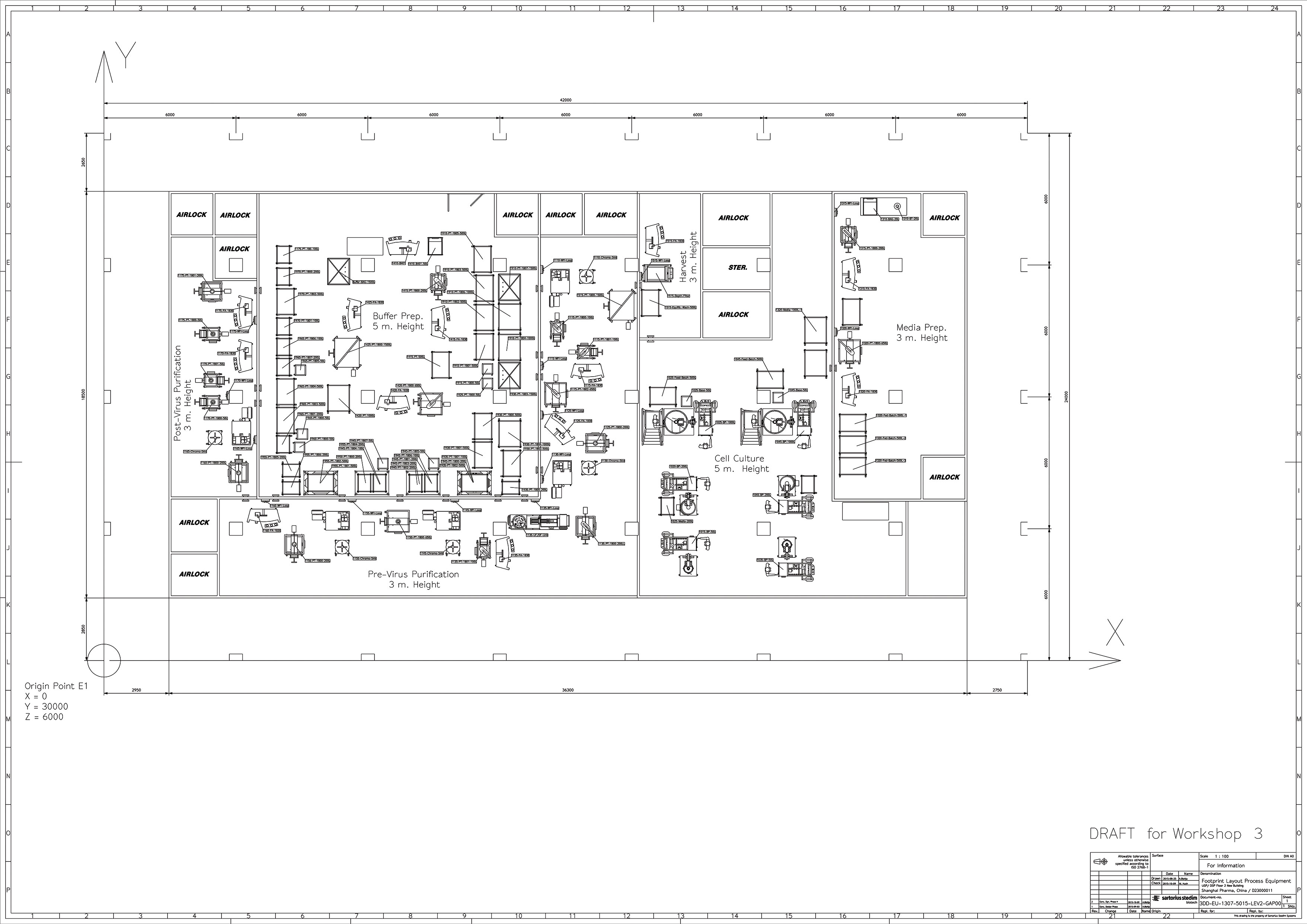
Figure 3. “U-turn” layout of a flexible mAb manufacturing facility
The logistics and handling of single-use systems are of course very different to hard-piped facilities, and for this reason the ergonomics and workflow require careful consideration. Horizontally positioned production operations located on one floor with sufficient space for material and personnel transfer represent the most suitable layout for single-use production facilities. These allow for bag-based transport in addition to the storage of raw materials and semi-finished and finished products.
With single-use process trains, we take full advantage of the opportunities to simplify material transfers, simplify circulation space, simplify material management, and downsize water and utility requirements (on average 70–80% less water is required for a fully single-use process train) so that end-users can claim a truly sustainable facility.
Successful project execution requires not only consideration of the bioprocess equipment, but also its automation and integration into a given IT infrastructure. Yet there is no unique standard in the pharmaceutical industry, and many customers are using a variety of components (sensors, actuators, local controllers, DCS, SCADA, etc.) from different vendors. Once the user has clearly defined requirements, automation engineers are able to translate these into a functional specification and afterward into a hardware and software design specification followed by a design qualification phase.
In parallel, we offer our customers different automation architecture options. This can be based on either our proprietary solutions (DCU, MFCS) or on industrial solutions (Siemens, Emerson, Rockwell) or even, where appropriate, a mixture of the two. In this way, Sartorius offers full flexibility in automation and can offer the most suitable solution for our customer’s requirements. We are able to create and build the solution and carry out the respective testing accordingly.
Some suppliers have developed modular facility concepts. For example Sartorius’ G-Con PODS5 or KeyPlants’ modular containers offer flexible modular systems that are perfect examples of how to enable the industry to cope with the challenges of the future. Figure 4 illustrates the application of such technology to a typical mAb facility equipped with Sartorius’ Process4Success single-use process platform technologies.
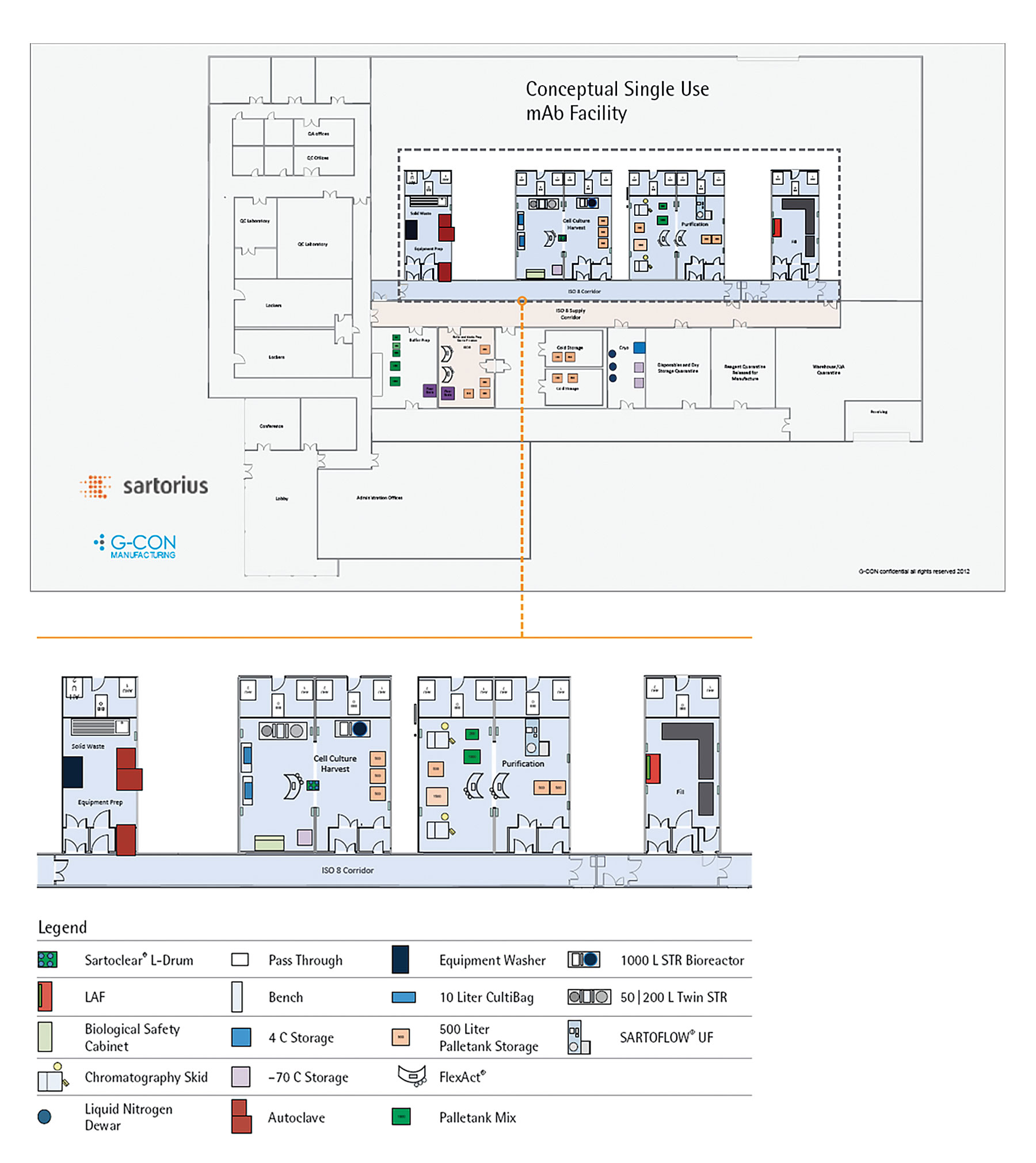
Figure 4. Modular facility based on G-Con PODs equipped with Sartorius Process4Success mAb platform process5
Thorsten Peuker, Ph.D. ([email protected]), is vice president, design & implementation, integrated solutions at Sartorius Stedim Biotech.
References
1. Breen, J.A., Chew, R.E., Kranking, L., Moelgaard, G. 2016. What is the Facility of the Future? Special Report: Facility of the Future. Pharmaceutical Engineering. Vol. 36, No. 3.
2. Langer, E.S. 2016. 4 Key Trends in Biomanufacturing in 2016. (Based on a report from BioPlan Associates Bioprocess Online.)
3. www.sartorius.com/sartorius/en/EUR/integrated-solutions/client-case-studies
4. Peuker, T., Monge, M. 2015. Implementing Flexible, Scalable, and Cost-Efficient Bioprocess Platforms: A Proven Project Management Approach. BioProcess Int. Vol. 13, Suppl. 1: 48–52. Informa Life Sciences Group.
5. Manzke, C., Diel, B., Peuker, T. 2014. Flexible Biomanufacturing Processes That Address the Needs of the Future. Adv. Biochem. Eng. Biotechnol. 138: 207–237. DOI: 10.1007/10_2013_220. Springer-Verlag Berlin Heidelberg.