January 1, 2010 (Vol. 30, No. 1)
Scientists in Academia Are Pushing Young Field Ahead with Diverse Range of Applications
The field of stem cell research is progressing rapidly despite technical and policy challenges. In fact, the global stem cell market is estimated to reach $88.3 billion by 2014, with the U.S. holding 60% of the market, according to a 2009 report from Bharat Book Bureau.
Although U.S. embryonic stem cell research guidelines have been relaxed since President Obama’s Executive Order this past July, several states (South Dakota, Louisiana, Arizona, Texas, and Oklahoma) are trying to restrict or altogether ban these efforts. In addition, there is still no federal law that prohibits reproductive cloning in the U.S.
In the U.K., however, such guidelines were established in 1990 via the Human Fertilization and Embryology Authority (HFEA). This group grants research licenses to individuals applying to use human embryos and regulates all clinics performing reproductive medicine. In 2001, its permission guidelines for embryonic research were expanded to include efforts under way to better understand human development and disease and to create new therapies.
Stephen Minger, Ph.D., former professor and director of the stem cell biology lab at King’s College, London, was the first researcher to obtain an HFEA license under these guidelines and reportedly the first to generate a human embryonic stem cell line in the U.K. Currently head of R&D for cell technologies at GE Healthcare, Dr. Minger’s academic work focused on developing cell therapy for diseases like multiple sclerosis, heart failure, and retinal disorders. The group utilized embryos with genetic mutations to develop cell lines in order to study the molecular basis of diseases.
According to Dr. Minger, this approach has advanced induced pluripotent stem cells (iPSC), allowing the creation of disease-specific cell lines from anyone with a known genetic mutation. “This opens up a whole new arsenal of approaches we didn’t have before and is moving research away from animal models and more into cell-based human models,” he reported at Select Biosciences’ “Stem Cells Europe,” meeting, which was held recently in Edinburgh.
Dr. Minger said that, although stem cell research is a young field, there has been a lot of progress academically and commercially growing cells under better conditions and to greater scale. “We probably still don’t have optimal growth conditions and differentiation protocols are still under development.”
Questions remain as to whether it’s possible to generate cells of high enough purity and quality for drug screening or predictive toxicology. “What purity do cells have to be to constitute a good clinical product? Is 95% acceptable if you know what the other 5% are?”
Questions also remain regarding clinical development. “We need to develop automated systems and technology to monitor the cells, to be able to grow the trillions of cells required to take cell therapy into real clinical practice. It’s impossible to say we’ll be there in two years or ten years. We still have quite a long way to go.”
GE Healthcare and Geron entered into a global license and collaboration agreement in June 2009 to develop and commercialize cellular assay products derived from human embryonic stem cells for use in drug discovery, development, and toxicity screening.
Cartilage Repair
Researchers at the University of Bristol have developed a tissue-engineering method to effectively take stem cells from bone marrow and, using the right growth factors, drive cartilage cell formation. Several challenges remain, reported Anthony Hollander, Ph.D., professor of rheumatology and tissue engineering. Since the patients are not life-threatened but in pain, and perhaps somewhat disabled, the burden of evidence that stem cell therapy is going to be safe and effective is much higher. Cost is another factor. “Although we’re using the patient’s own cells and there is no immune rejection, it’s personalized medicine and very expensive.”
Dr. Hollander’s research is focused in two main areas. The first is growing cartilage from adult stem cells to treat osteoarthritis in aging individuals. It takes approximately 48 hours to turn adult stem cells into cartilage cells, compared to five weeks to create a piece of cartilage from tissue culture. This is a long way from clinical applications, he added, because they still have to discover how to grow large pieces of cartilage and how to integrate them with the surrounding natural cartilage in the joint.
Another research area is repairing torn meniscal cartilage, the semicircular cartilage pieces in the knee that act as shock absorbers. In most cases, the only treatment is surgery, which later increases the risk of osteoarthritis. Dr. Hollander’s group has developed a stem cell bandage for which stem cells are taken from the patient’s bone marrow and grown in culture. These are kept on a scaffold as undifferentiated stem cells until they reach the required amount.
“We place these cells in the middle of the torn meniscus and sew it up around the cell bandage. The idea, and we’ve shown this in the lab, is that the stem cells migrate between the two surfaces and literally knit across the surface, causing an integration.”
A spin-out company, Azellon Cell Therapy, has been created to market the Cell Bandage, which is currently being tested in sheep. According to Dr. Hollander, the company has funding for a safety study to start next year in 10 patients. The next big step, he said, is to scale up these techniques, to increase implant size and develop a donated cell method in order to use cell lines to treat large numbers of patients.

Researchers at the University of Bristol grew cartilage from undifferentiated adult bone marrow stem cells.
Re-Myelinating Nerves
“Myelin disorders have a lot of appeal versus other diseases like Parkinson, where you are trying to replace dead and/or dying neurons,” stated Ian Duncan, Ph.D., professor of neurology, school of veterinary medicine at the University of Wisconsin, Madison. “It’s rather straightforward with myelin disease—we know myelin sheaths are replaceable if you have the right cells.”
The biggest challenge is isolating sufficient cells capable of replacing myelin in focal lesions in significant numbers of patients. Dr. Duncan said it’s an issue of cell cultivation. “Although things are improving technically, we’d like to be able to make oligodendrocytes in large numbers in culture before taking the cells into an intermediate stage. We’d like to have confidence in vitro that there’s significant differentiation toward those cells prior to trying it in vivo.”
He added that the derivatives of embryonic stem cells have not been as successful as those of human neural crest stem cells. But they are not ruling out using induced pluripotent stem cells or oligodendrocyte progenitors as potential cell sources.
Another goal is to repair focal areas of damage that are strategically significant. Dr. Duncan’s group would like to deliver cells in a “disseminated way throughout the entire CNS, but that’s a big challenge. It’s unlikely that you could put cells into the spinal cord and have them migrate to the brain.”
Dr. Duncan said that he has demonstrated re-myelination of a damaged segment of spinal cord in a mouse model. He added that although they have a better idea of how to make a lot of oligodendrocyte progenitors from human embryonic stem cells, they still haven’t obtained the quantity and good differentiation potential they would like.
Epidermal Neural Crest Cells
Epidermal neural crest cells (EPI-NCSC) are remnants of the embryonic neural crest and give rise to hair, skin, sweat glands, peripheral nerves, facial bones and cartilage, pigment, and some endocrine glands. Maya Sieber-Blum, Ph.D., professor of stem cell science at Newcastle University, said that some of these cells survive in hair follicles, where her group discovered them by accident. “When we saw these neural crest cells in the bulge of hair follicles, we first had to prove they were stem cells.”
The cells were molecularly characterized by long serial analysis of gene expression (longSAGE). Data showed that 19 genes are characteristic for both embryonic neural crest stem cells and EPI-NCSC, but are not expressed by epidermal stem cells. These 19 genes were validated at the protein level by immunocytochemistry. “We used that molecular signature to compare it to other published data to find out if our cells are unique—and they are unique among skin resident stem cells.”
One of the challenges in working with human EPI-NCSC is expansion in cell culture. Dr. Blum said her lab has developed a technique to efficiently accomplish this, but was unable to provide details due to IP restrictions.
In another study, Dr. Blum wanted to see if her cells expressed the same four genes that are used for induced pluripotent stem cells. Data shows that Myc, Sox2, Klf4, and Lin28 are expressed at levels similar to mouse embryonic stem cells. However, the pluripotency genes Nanog and Oct4 are expressed at 700- to 800-fold lower compared to embryonic stem cells. “This likely explains the fact that EPI-NCSC did not form tumors in the spinal cord of mice.”
In collaboration with the Brain Research Institute, the cells were tested in mice with spinal cord injuries. Data showed integration into the spinal cord, cell survival in high numbers, vascularization of the scar, bridging of the lesion, and no tumor formation. Potential therapeutic applications include spinal cord injury, repair of neural degeneration, as well as bone transplants.
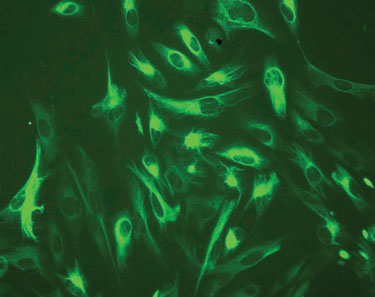
Nestin is a stem cell marker that is expressed in epidermal neural crest cells. (Newcastle University)
Skeletal Muscle Cells
Understanding how skeletal muscle stem cells function may provide information leading to treatment of, not only muscle damage but also muscle wasting, often associated with cancer, HIV, cardiac cachexia, and rheumatoid arthritis. Claire Stewart, Ph.D., professor of cell and molecular biology, Manchester Metropolitan University, has been studying regulators of skeletal muscle degeneration and regeneration.
Initial challenges in working with these cells involved developing and establishing cultures. Dr. Stewart’s group developed specialized media to ensure performance of growth factor/cytokine studies. “Surprisingly, these are really great cells to work with—they grow and behave well in culture.”
Another hurdle is the construction of experiments. “We all have our favorite growth factors of choice alongside signaling molecules and cellular responses of choice, e.g., we are taking a linear approach to our studies. We need a multivariate approach that provides greater opportunity in that vaguely interconnected and simultaneous random observations are very often related.”
According to Dr. Stewart, this would provide unbiased insight into the mechanisms governing cell behavior. However, these studies are often viewed as fishing expeditions and usually not funded.
Recent research efforts have resulted in some exciting discoveries. The area Dr. Stewart calls “the most exciting work we’re doing at the moment,” involves cell memory and aging. Muscle biopsies were taken from controls and cancer patients to compare the number of stem cells. Results showed an age-related decline in muscle stem cells but no decline was seen in cancer patients.
“A 70-year-old cancer patient had the same number of muscle stem cells as a 40-year-old, but this wasn’t seen in the control population.” Dr. Stewart explained that it appears that biopsy tissues retain memory of their in vivo environment. “This has implications for all autologous stem cell research, making tissue sources and host environments key to autologous treatment.”
Additional efforts are being focused on multivariate analysis and development of muscle stem cell markers, 3-D cultures and regulation of cell fusion, improving computer tracking studies in injury, and establishing virtual muscle stem cell models.
There’s little doubt that ongoing stem cell research will continue to discover potential new therapies for a wide range of diseases as well as novel drug screening assays. Advanced Cell Technology recently filed an IND with the FDA to use embryonic stem cells to treat Stargardt’s macular dystrophy—a photoreceptor degenerative disease that leads to blindness. This will be the second U.S. company, providing the FDA approves, to test human embryonic stem cells in patients.



