November 1, 2009 (Vol. 29, No. 19)
Myriad of Compounds Intended to Stop the Progression of Metabolic Diseases Moves Through the Pipeline
The competition to develop new therapeutics targeting metabolic disease is heating up. Here’s why: the latest estimates from the American Diabetes Association state that there are nearly 24 million Americans with diabetes. In addition, approximately 32% of American adults are medically obese.
Many companies have honed in on this large and growing market, and several of them presented their latest findings at IQPC’s “Groundbreaking Advances and Key Opinions in Metabolic Diseases Drug Discovery and Development” held recently in San Francisco.
“When we founded the company, we wanted to work on the biology responsible for disease progression,” stated Teo Uysal, president and CEO of Syndexa Pharmaceuticals. The company is now focused on endoplasmic reticulum (ER) stress and inflammatory pathways. Uysal said there is growing evidence that ER functional capacity is important in disease progression. If its capacity or homeostasis is compromised, the related pathways eventually have a negative effect on insulin signaling in peripheral tissues and macrophage function.
“ER, in its functional capacity, appears to be at a very critical junction in biology. Its modulation seems to have a therapeutic benefit in multiple indications,” explained Uysal. Animal studies support this concept. If one can alleviate the cell stress, he added, one will observe profound antidiabetic and antiatherosclerotic effects.
The company has developed technologies to study this organelle, some of which are partially licensed from its scientific founder’s lab (Gokhan Hotamisligil, M.D., Ph.D.) at Harvard University. Using systems biology drug-screening capabilities, the company has been able to monitor the adaptive functional capacity of ER, with the goal of identifying small molecules to enhance this function.
Syndexa is also researching a stress kinase, JNK (c-Jun-N-terminal), which plays a critical role in type 2 diabetes, as well as other metabolic diseases. The company’s approach is unique, according to Uysal, because it has chosen to use allosteric inhibition of the kinase by targeting the substrate-docking site, instead of the ATP binding site, thereby avoiding problems like cross-reactivity.

The SIRT1 enzyme is the most well-studied of the seven sirtuin family members. (David Shopper Photography)
Modified Lipid Biosynthesis
Researchers at Xenon Pharmaceuticals are developing inhibitors of SCD1 (Strearoyl coA Desaturase), the rate-limiting enzyme for production of mono-unsaturated fatty acids. “SCD1 is an interesting drug target because it seems to be at a junction point of how the body regulates whether or not lipids go into a storage or oxidation pathway,” explained Michael Winther, Ph.D., senior director, drug discovery. “That choice, that’s made in different tissues at different times, is fundamental to metabolic health and regulation of weight and fatty acid levels.”
The company has moved forward with studies because it has human data supporting the extrapolation of the rodent pathways. Rodents with SCD1 knock-out have a changed fatty acid composition that’s easy to measure. However, there is no known SCD1 knock-out in humans.
This presented its own challenges as there were no precedent in vitro or in vivo assays available. “The first challenge was HTS—there were no known small molecule inhibitors to inhibit SCD1 in the way we wanted our drug to inhibit it.” In addition, there was no crystallographic structure for the SCD1 enzyme, so computer-assisted drug design was of limited value. So, the researchers developed an HTS method for SCD1, which is also useful for screening related enzymes involved in fatty-acid metabolism, specifically other fatty-acid desaturase and fatty-acid elongase enzymes.
Another challenge in developing the model for SCD1 is the rodent models. Rodents have four SCD1 genes (SCD1, 2, 3, and 4), versus humans, which have SCD1 and SCD5. So rodent studies have to be treated with caution since one cannot replicate the SCD1 population that exists in humans. “If you knock out certain lipid pathways, you’re going to have the potential to run into problems. This is true for any lipid pathway targets, because they are essential for maintenance of health,” added Dr. Winther.
His group will continue its work on the basic biology of SCD1 and how it regulates the process in rodents, and then extend that information into humans. “A better understanding of how SCD1 regulates body weight and metabolism is the next stage for the field.”
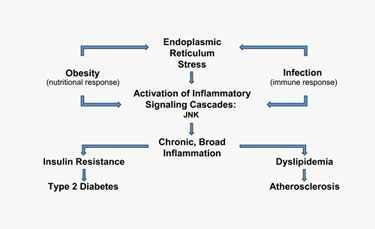
Activation of the overlapping metabolic and inflammatory signaling pathways causes an increase in ER stress and JNK activity.
Developing Sirtuins
There are seven human sirtuins—all with a different biology. One challenge is trying to understand which sirtuin to target for a specific disease. Sirtris Pharmaceuticals, a GlaxoSmithKline company, is focusing on identifying small molecule activators that are structurally different from resveratrol to activate SIRT1.
“What’s unique about SIRT1, versus other potential targets, is that it is really a stress-related sensor and only active in the case where the cell is under some type of stress—oxidative stress, DNA damage, or stress related to increased glucose levels,” stated George Vlasuk, Ph.D., president. He added that the company has focused most of its efforts on finding modulators of SIRT1 for metabolic disease.
Finding small molecule activators is difficult because there are few small molecules that can activate enzymes. The observation that reservatrol could enzymatically activate SIRT1, lead the company to develop assays to screen chemical libraries to, not only find molecules to act like resveratrol, but to specifically activate SIRT1. (There is data on resveratrol inhibiting other enzymes and binding to certain receptors). Some of these molecules have been studied in preclinical disease models.
Dr. Vlasuk said that additional challenges are validating molecules that are acting solely through the activation of SIRT1 in cells and tissues. “In some cases, we’ve demonstrated that clearly, and in other cases, we haven’t been able to due to technical challenges.
“We’re trying to understand what the relationship in regulation of SIRT1 is in various cell types. The question is how is this actually regulated in a cell and how does that impact how the small molecule can regulate or activate SIRT1 in a cell or tissue? That’s the fuzzy area now—where we’re trying to develop the biology to the point where we understand how these molecules are impacting SIRT1 in a cell, tissue, and, ultimately, in an animal.” The company’s lead molecule is currently in a Phase IIa trial in type 2 diabetes.
Inhibiting Triglyceride Synthesis
DGAT1 (diacylgylcerol acyltranferase) is the enzyme that catalyzes the final step in triglyceride synthesis. The advantage of DGAT1 inhibitors, said Robert Dow, Ph.D., associate research fellow, CVMD chemistry at Pfizer, is the potential for positive effects on triglyceride levels, especially in the liver, with “a profound effect on normal functioning of insulin action and glucose utilization.”
Animal studies have shown a substantial reduction in triglyceride content in rodent livers, as well as good plasma clearance and a good projected half-life of eight hours. A subsequent single-dose study in healthy male volunteers showed these predictions were good, and based on this initial data, the company plans to go into multidose and proof-of-mechanism studies.
Previous animal studies with DGAT knock-out mice showed highly reduced levels of triglyceride content in the liver, adipose, and skeletal muscle. These animals lose weight and become resistant to further obesity due to a high fat diet. “That was part of the rationale that got us excited about this approach, knowing we would have an impact on diabetes end points, as well as, potentially, obesity endpoints,” stated Dr. Dow.
In order to avoid losing compounds in later stages of development, his group applied its de-risking strategy in early lead optimization. “The next frontier of drug discovery is to drive safety assays early into the discovery process, so we can head off the sorts of problems where we are losing compounds in later stages of development.”
His group used NMR technologies to understand whether they were forming glucuronides, which can often rearrange and interact with proteins in the body to create toxicities. The results showed low amounts of rearrangements and, therefore, low risk. “Rather than waiting for something to happen in a half-percent incident in humans in a Phase IV study, where you’d need thousands of patients to pick up such a small percent, we move it to the discovery phase and derisk it.”
There were also concerns regarding whether the lead compound had photo instability. Dr. Dow said one of their assays looks at cytotoxicity of the compound in the presence and absence of light. The lead compound did show cytotoxicity in the presence of light, but a modified molecule showed no toxicity. Overall, these steps are part of the company’s approach to move initial in vivo tolerability studies early into their lead-development program.
Developing a new therapeutic for diabetes is challenging due to the progressive nature of the disease. “The bottom-line challenge for all of us is to identify compounds that have not only the initial effect of blood glucose lowering, but that will have positive, long-term effects for diabetics.”
GPCRs
GPCRs (G-protein coupled receptors) account for a large percentage of drugs being developed. Researchers at Arena Pharmaceuticals conduct their lead development around the hypothesis of stabilizing the constituitively active form of GPCRs to generate a signal without having to identify the natural ligand prior to discovering a small molecule to that receptor.
This is the basis of their technology CART—constituitively activated receptor technology—which enhances detection and allows simultaneous identity of the receptor inhibitor and activator drug leads.
The company’s initial lead molecule, GPR119, was an inverse agonist. “We used medicinal chemistry,” explained Dominic Behan, Ph.D., cofounder and CSO, “and flipped the activity of the inverse agonist to an agonist. The initial breakthrough was getting a molecule interacting with the receptor, and then designing agonists around that scaffold.”
Another technology uses melanophores, frog skin cells, to measure activity stimulated by GPCRs and by small molecules that target those GPCRs. The pigment in the frog cells will disperse within the cell if it stimulates cyclic AMP, and this is measured by absorption. “This was used in the early discovery of GPR119 and was later evolved into different assays,” said Dr. Behan.
James Leonard, Ph.D., senior director, metabolic disorders, presented the company’s approach to discovering and developing GPR119. Using the firm’s technologies, he said they found that this molecule increased cyclic AMP in insulin-producing cells of the pancreas. “Here we have a novel receptor in the cell type of interest, and it signals in the same way as a receptor (GOP1) with a nontherapeutic benefit. So the strategy was, can we find small molecules and replicate the same biology? In short, that’s what we did.”
Additional research showed GPR119 expression not only in the pancreas, but also in the intestinal endocrine cells (stimulating release of GLP1, GIP, and PYY—all hormones that are known to be beneficial in the regulation of glucose homeostasis, and which are positively regulated by GLP119).
Dr. Leonard said these studies have shown GLP119 has positive effects on oral glucose tolerance and maintains activity when given repeatedly, in addition to showing chronic efficacy in rodent models of type 2 diabetes, with lower HDA1C, improved glucose, and lipid profiles.
There’s little doubt that the global markets for prescription endocrine and metabolic disease drugs will increase. A 2007 report by BCC Research estimated the market would reach $96.4 billion by 2011, up from $72.3 billion in 2006. As research moves forward, there is a great deal of promise for new therapeutics that may not just address the symptoms of metabolic disease but stop disease progression.



