November 1, 2010 (Vol. 30, No. 19)
Long Shelf Life Gels Designed to Improve Consistency, Speed, and Reproducibility
In a paper published online August 22 in the journal Nature, Ryan Jensen, Ph.D., postdoctoral researcher at the University of California, Davis, and his colleagues reported the first purification of full-length BRCA2 protein. The protein, mutations of which lead to breast and ovarian cancers, has eluded research groups for years due to challenges preventing its purification.
Like many researchers, Dr. Jensen used his own handcast polyacrylamide gels to monitor the purification of BRCA2. During many of these attempts, the higher molecular weight proteins—including BRCA2—were undetectable.
When Dr. Jensen began using Bio-Rad Laboratories’ Mini-Protean TGX long shelf life gels, he consistently observed “tight, crisp” bands corresponding to BRCA2. “When I hand poured my gels to analyze the purification of BRCA2, I wasn’t always confident that it would show up,” Dr. Jensen said. “With Bio-Rad’s TGX gels, I can count on the fact that BRCA2 will show up every time.”
Since the late 1960s, the SDS-PAGE workflow has become a widely used tool for analyzing protein mixtures. Within that workflow, the Laemmli system is regarded as the gold standard for SDS-PAGE techniques. Utilizing a Tris-glycine-SDS buffer system, the Laemmli system has the ability to clearly resolve and provide accurate molecular weight estimation of proteins in complex samples from a wide variety of sources.
Researchers looking to use the Laemmli system have two options for gels: handcast gels and precast gels. Handcasting polyacrylamide gels is time-consuming and often leads to inconsistent results. Handcast gels must also be used immediately. In their favor, handcast gels run on the standard Laemmli buffer system and serve as an inexpensive option for cost-conscious labs.
Commercially available precast gels, on the other hand, save researchers from the time and hassle of handcasting. However, precast gels with longer shelf lives typically require the use of alternative buffer systems such as MOPS or MES that present different electrophoretic patterns, affecting both the resolution and banding patterns of proteins in complex mixtures.
Specialized buffer systems also usually cost more per run, so given the widespread use of SDS-PAGE, this can have a significant adverse impact on a laboratory’s budget.
Precast gels that utilize standard buffers face the disadvantage of the traditional Laemmli systems’ loss of gel matrix stability, resulting in a shelf life of only a few months. Gel performance steadily degrades over time, reducing resolution and sensitivity, and increasing time-to-results.
In response to these and other challenges, Bio-Rad introduced Mini-Protean TGX long shelf life gels. These long shelf life gels are based on a novel modification of the Laemmli system, and offer advantages over both handcast and other precast gels in terms of consistency, speed, and reproducibility.
Performance
TGX gels retain superior Laemmli-like separation characteristics using standard sample and Tris-glycine running buffers. The patented modification of the Laemmli buffer system has reduced hydrolysis of the gel matrix over time, thereby extending shelf life up to 12 months and delivering consistent, high-quality performance.
At 300 V, a typical run can be completed in 15 minutes (compared to 30–45 minutes for other precast and handcast gels). This is because TGX gels can be run at higher voltages than other gels without impacting performance (Figure 1). The subsequent transfer step can be completed in 15 minutes at 150 V (compared to 1 hour to overnight).
This new gel technology delivers uniform band shape and symmetry—lot-to-lot and intra-lot reproducibility ensure consistency of results.
“I think there’s a pervasive feeling among researchers that their own hand-poured gels have better resolution, but I would argue the opposite,” said Dr. Jensen. “The TGX gels provide a facile solution for producing publication-quality figures.”
Mini-Protean TGX gels demonstrate greater linearity of separation, which results in increased MW estimation accuracy when compared to traditional Laemmli precast gels or NuPAGE Bis-Tris gel systems. A linear regression analysis of estimated MW vs. known MW yields a coefficient of linearity (R2) of greater than 0.98 for TGX gels, vs. an R2 of only 0.86 for a representative NuPAGE gel.
Dynamic range of quantitation and resolution are also superior with TGX gel technology. TGX gels exhibit greater lane and band symmetry than traditional Laemmli gels or alternative long shelf life gels, even when overloaded.
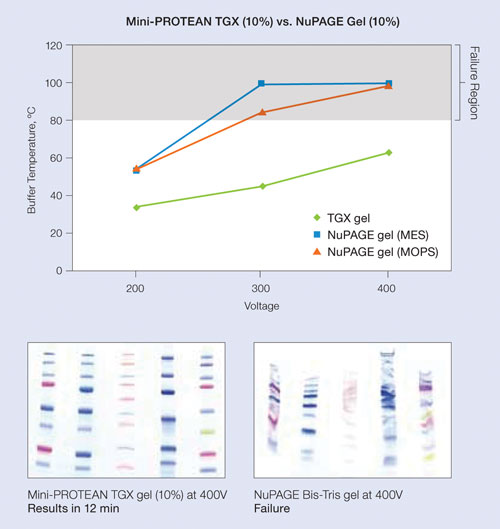
Figure 1. Performance at elevated voltages: Various Precision Plus Protein standards were loaded on either a 10% Mini-Protean TGX gel or a 10% NuPAGE gel and run at 200 V, 300 V, and 400 V. Buffer temperature was measured at each voltage; NuPAGE gels generate excessive heat at 300 V or above, negatively impacting performance, while the Mini-Protean TGX gels maintain lower temperatures at elevated voltages and provide superior performance.
Downstream Applications
The Mini-Protean TGX gels deliver superior staining with low background. These gels are compatible with all commonly used stains and mass spectrometry applications. Dr. Jensen remarked that using Bio-Rad’s precast gels prevented user contamination that could potentially interfere with mass spectrometric analysis when using handcast gels.
The Mini-Protean TGX precast gels provide transfer efficiency with both wet/tank and semi-dry transfer systems. The proteins from the gel can be transferred onto a PVDF or nitrocellulose membrane in as little as 15 minutes (Figure 2).
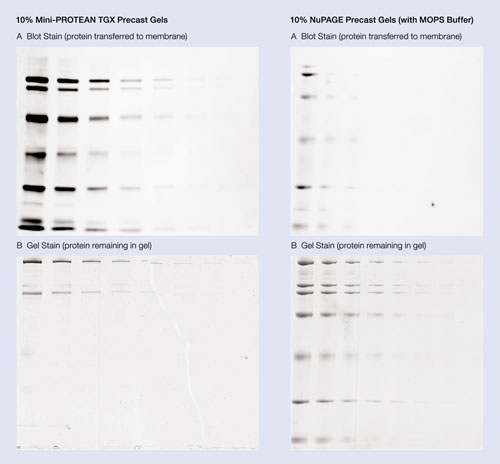
Figure 2. High transfer efficiency of Mini-Protean TGX precast gels: Broad range protein standards were prepared as 50-fold dilutions in Laemmli sample buffer for precast TGX gels or NuPAGE sample buffer for NuPAGE gels. Serial twofold dilutions of this sample were loaded on the 10% precast gels in a volume of 5 µL. Gels were run using Tris/Glycine/SDS buffer for TGX gels or MOPS buffer for NuPAGE gels. The proteins from the gel were then transferred into nitrocellulose membrane in a Mini-Protean Tetra cell using the Mini Trans-Blot® module at 110 V (constant voltage) for 15 minutes with prechilled Towbin buffer. Protein transfer was evaluated by staining the membrane with SYPRO Ruby blot stain and imaging on the Molecular Imager® VersaDoc™ 4000 instrument with a three-second exposure (A). Proteins remaining in the gel following transfer were visualized by staining with Bio-Safe Coomassie stain and imaging with the Molecular Imager GS-800 densitometer (B).
Conclusion
TGX gels are currently available in 7.5%, 10%, 12%, 4–15%, 4–20%, and an Any kD gel formulation. Any kD gels offer resolution of proteins in the 10–250 kD range and can be used in screening experiments. These gels deliver superior resolution in the 20–100 kD range, the range most evaluated in 2-D samples, making it ideal for 2-D applications. Furthermore, all precast gels offered by Bio-Rad are manufactured without SDS and therefore, can be used for native PAGE applications.
Top Ten Tips to Improve Protein Gel Electrophoresis
Over the last 15 years, Aldrin V. Gomes, Ph.D., assistant professor at the University of California, Davis, and his colleagues have routinely used gel electrophoresis in many of their experiments. Here are their top 10 tips that will help ensure great protein gel electrophoresis results:
1 For the best resolution, protein samples should be vortexed before and after the heating step.
2 Samples should not be boiled at 100ºC. Samples boiled at high temperatures can lead to high molecular weight aggregates. Samples should be heated at about 70ºC but less than 95ºC for 3–5 minutes for an SDS sample buffer preparation.
3 Add 10 µL of 1x sample buffer to unused well lanes to avoid gel edge effects.
4 SDS-PAGE running buffers can be reused two times without higher background signals. Reusing the buffer more than two times is possible, but running time increases.
5 Sample loading buffer is important for the final resolution of samples. The most common mistake is not adding enough bromophenol blue making it difficult to see the sample when it is being loaded into the wells. To improve reproducibility and reliability, make 100 mL of Laemmli Sample Buffer and aliquot this into 1 mL fractions. Once validated, the batch of buffer solution can be used for over 500 gels. Sample buffer aliquots are stable for 6 months at -20ºC and >1 year at -80ºC.
6 Only constant voltage gives constant protein mobility during gel electrophoresis, NOT constant power conditions.
7 Double-check wells for damage and note the maximum volume that the well can hold. Overfilling wells can lead to artifacts.
8 Remove aggregates and improve resolution by centrifuging all samples in a microfuge at >10,000 xg for two minutes prior to loading.
9 Samples boiled in sample buffer can be aliquoted and stored at -20ºC for >4 weeks or at 4ºC for at least a week. Samples should be warmed at 37ºC for 1–2 minutes before
use. Repeated freeze-thawing can lead to protein degradation.
10 High salt concentrations cause gel artifacts. If the salt concentration of a sample is high, the protein can be concentrated with 10% (w/v) trichloroacetic acid (TCA) (incubate for five min. at 4ºC). The precipitated protein is collected by centrifugation and the pellet washed with cold acetone. The pellet is then resuspended in an appropriate buffer. If the sample concentration is too dilute, then TCA could also be used to concentrate these samples.
For more detailed tips, visit www.lablife.org/lab?groupid=2441 for a Protein Gel Electrophoresis Tips and Troubleshooting Guide.
Christopher Belisle, Ph.D., is marketing manager of electrophoresis and blotting, and Kate Smith ([email protected]) is senior product manager, electrophoresis, both in the laboratory separations division at Bio-Rad Laboratories.



