September 15, 2009 (Vol. 29, No. 16)
Automation Helps Remove Pipetting Bottleneck and Avoids Mechanical Stress
High-throughput cell-based assays are an integral part of the drug-screening process. With the ability to automate and subsequently accelerate the entire process, high-throughput screening (HTS) allows researchers to conduct biochemical assays to rapidly identify active compounds, antibodies, or genes of interest. The results of such screening experiments provide the starting point for drug design as well as an understanding of various cellular interactions.
With the ability to screen a wide range of static assays against a library of candidate compounds, HTS provides analysis for cellular events such as kinase activation/inhibition, up/down regulation of signal transduction pathways, and apoptosis, to provide a detailed understanding of potential drug effects. The assays that are incorporated into any one screening campaign are dependent on the therapeutic targets, which can include cancer, cardiovascular disease, and inflammation. However, for any assay, an accurate method of dispensing live cells is essential and must be suitable for all assay types.
Successfully dispensing cells while maintaining viability is essential to the entire procedure in order to ensure that accurate resulting data is obtained. Traditionally, researchers have used conventional hand-held pipettes to enable gentle dispensing that does not cause any damage, ensuring that cell viability is effectively maintained.
Although this is effective, pipetting is often viewed as a bottleneck due to its time-consuming, labor-intensive process. Automated reagent dispensers can be used to achieve a higher throughput within screening laboratories. Dispensing in this manner must also be gentle so as not to cause any damage to the cells.
In this article, we investigate the process of dispensing live HeLa S3 cells into 96- and 384-well microplates using the Thermo Scientific Multidrop Combi reagent dispenser from Thermo Fisher Scientific. The results are subsequently compared with cell viability data achieved using hand-held pipettes. The peristaltic pump technology and optimized dispensing cassettes of the Multidrop® Combi provide the capability to quickly dispense cells into any multiwelled plate type for all varieties of cell-based assay.
Culturing the Cells
In this investigation, HeLa cells were used since they are a well-known and commonly used cell line with an innate ability to proliferate at an extremely rapid rate. As such, these cells are often used in a number of different high-throughput cell-based assays. The HeLa S3 cells were grown in adherent culture (Kaighn’s media with fetal bovine serum and penicillin/streptomycin) using standard culture flasks in a CO2 incubator at 37°C with 5% CO2. The cells were maintained at 0.2–0.6 x 106 cells/mL by passaging 1:5–1:8 every three days.
Detaching and Dispensing
Enzyme-induced dissociation with trypsin was used to remove and collect the cells from the flask. Commonly used in cell culture labs, trypsins are often added to cleave the peptide chain linking the cells to the surface of the culture flask, dish, or plate. The number of cells collected was determined, and the Multidrop Combi was used to dispense both the cell suspension and reagents into cell culture plates. The tubing was prewet with phosphate buffered saline and primed with the cell suspension, before dispensing the samples. When pipetting into a number of different plates, the cassette was primed before each plate.
The standard tube-dispensing cassette was used to dispense 100 µL of the cell suspension into 96-well plates, and the small tube plastic tip dispensing cassette to dispense 30 µL into 384-well optical bottom plates. As a result, concentrations of 5,000 cells per well and 2,000 cells per well were observed in the 96- and 384-well plates, respectively.
As a control measure, an equal number of cells were hand pipetted into the second to last column of the plates, and growth media was dispensed as a blank control. To ensure accuracy and repeatability, cells were dispensed in four replicate plates for both plate types.
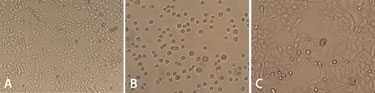
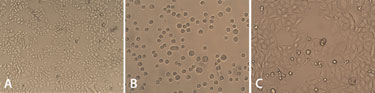
Figure 1. (A) HeLa S3 cells attached to the bottom of the cell culture flask, (B) in suspension immediately after being dispensed, and (C) 24 hours after adherence to the 96-well plate.
Cell Viability
After dispensing, cell viability was tested using a proliferation assay (CellTiter 96® Aqueous One Solution Cell Proliferation Assay from Promega). The assay is based on bioreducing the tetrazolium compound into a colored formazan product, which is directly proportional to the number of living cells present. Before dispensing the cells, the assay reagent was dispensed using the Multidrop Combi in combination with the small tube plastic tip dispensing cassette. 20 µL/well was dispensed into the 96-well plate, and 6 µL/well to the 384-well plate. Both plate types were incubated for two hours at 37°C prior to measuring absorbance at 490 nm in a microplate photometer.

Figure 2. (A) Dispensing of the cell suspension on the 96-well plate (B) Dispensing of the cell suspension on the 384-well plate.
Results
Before dispensing, the adherent HeLa S3 cells were examined under a microscope and were found to be viable, as seen in Figure 1A. After detachment with trypsin, the number of cells present were calculated and subsequently dispensed into microplates, again using the Multidrop Combi.
The cells in suspension were then observed under the microscope immediately after dispensing Figure 1B. These cells were viable and completely intact, with no visible damage. Following further cultivation, the cell culture adhered to the microplate where it continued to grow evenly—Figure 1C shows the cells 24 hours after adhering to the surface of a 96-well plate.
The cell proliferation assay demonstrated that the levels of viability for the cells dispensed using the Multidrop Combi and those dispensed with the manual pipette were the same. When using the standard tube-dispensing cassette, an even dispense is obtained across the 96-well plate, as seen in Figure 2A. The results shown are averages of all the wells in each column. Figure 2B demonstrates the same even distribution when using the small tube plastic tip dispensing cassette. Cell viability is comparable to hand pipetting with both cassettes and plates.
Conclusion
The cell viability assay demonstrates that the Multidrop Combi reagent dispenser is suitable for handling live cells. The cells remained intact and viable after automated dispensing, with no indications of any mechanical stress. Both the standard and small tube plastic tip dispensing cassettes demonstrated the precision with which cells can be dispensed. In order to obtain reproducible cell dispensing results, the cassettes require regular maintenance to avoid any clogging of the tube or tip.
Sini Suomalainen ([email protected]) is application
scientist at Thermo Fisher Scientific. Web: www.thermo.com.