July 1, 2011 (Vol. 31, No. 13)
Standardized Guidelines Gradually Taking Form as Emerging Discipline Moves Forward
The pioneering status of regenerative medicine is forcing developers and regulators to write the rules as they go, resulting in a highly collaborative regulatory environment that builds the framework for all subsequent regenerative therapies. Speakers at the recent “World Stem Cells and Regenerative Medicine Congress” explored that situation, highlighting many of the therapies in clinical trials now and some of the lessons they have learned.
Dendreon paved the way through many of the regulatory hurdles, winning approval in April last year for the first autologous cellular immunotherapy. “That set a precedent approval pathway,” notes Tim Mayleben, president and CEO of Aastrom Biosciences.
Nonetheless, the dearth of approved cellular therapies continues to present challenges and opportunities. “There are no hard and fast rules, so working with the FDA requires a more collaborative process,” Mayleben said. “Approach with sound data and work with the regulators there as scientists and colleagues.”
“Cell therapies are, necessarily, establishing the regulatory guidelines as they go,” said Kristin Comella, CSO for BioHeart. “Because we have a less formal relationship with our clinical reviewer at the FDA, we can call or email a quick note to get guidance about a particular approach or other issue,” she said.
BioHeart’s MyoCell® is a case in point. It reportedly improves cardiac function by populating regions of scar tissue within a patient’s heart with myoblasts derived from a biopsy of a patient’s thigh muscle.
MyoCell is developed from muscle-derived stem cells and can only form muscle, Comella emphasized. Therefore, they are used to help congestive heart failure patients form muscle to improve cardiac function. “They are delivered with a catheter through the femoral artery directly to the heart, so there is no need to crack open the chest,” she continued.
It is one of the few stem cell therapies currently in Phase III trials in the U.S. “Yet, stem cell therapies fall under pharmaceutical procedures,” Comella says, “which requires demonstrating potency. That is an almost impossible challenge for live cells,” she explained.
That sort of challenge is one of the reasons MyoCell’s development process has been so lengthy. “We conducted our Phase I study for MyoCell in 2001, and have spent more than $100 million, yet we still don’t have a commercial product.”
She suggested that a commercialization pathway should be developed to deal with autologous therapies, which are based upon a patient’s own cells, and to facilitate process innovation during development without returning the project to animal trials.
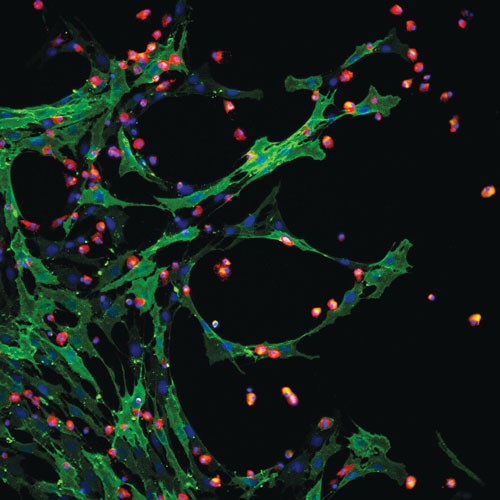
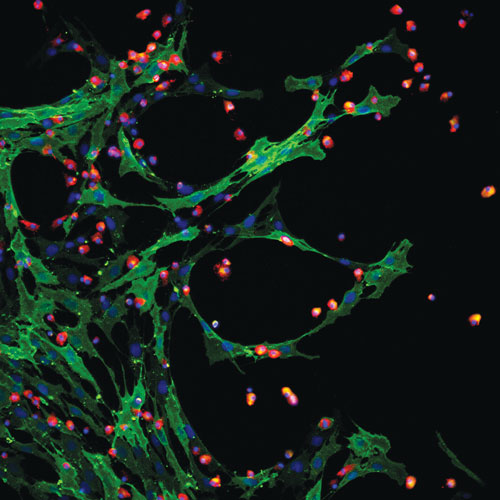
Aastrom Biosciences’ multicellular therapy contains mesenchymal and hematopoietic stem cells.
Multicellular Therapy
Mayleben outlined the development of Aastrom’s lead product, known generically as ixmyelocel-T, an autologous, expanded multicellular therapy derived from bone marrow.
“It’s unique in that we start with the patient’s own bone marrow—the body’s reservoir of stem and progenitor cells. It is expanded, so while we start with a very small aspirate, we significantly expand the number of important CD90 and CD14 auto+ cells.” And, he continued, “it is multicellular, containing many different types of cells, rather than only selected single cells such as mesenchymal stem cells. Therefore, it has multifaceted mechanisms of action.
“Bone marrow has been used successfully for 30 years, so it is well-characterized, safe, and efficacious, but unpatentable,” Mayleben said. Aastrom has built upon that record by expanding and enriching the cells and developing a patentable version.
Aastrom anticipates beginning Phase III trials in no-option critical limb ischemia (CLI) patients during the third quarter of 2011. The company has fast-track designation for the CLI program and is completing negotiations with the FDA for a special protocol assessment for the Phase III program.
Phase I trials were conducted in Germany, which, Mayleben said, “historically has had broader acceptance of cellular therapies and easier rules to navigate for deriving clinical data for experimental therapies.” That data was presented to the FDA before beginning later-stage U.S. trials.
The use of autologous cells also streamlined development. As Mayleben explained using a bread-baking analogy, “We’re not scaling up from a one pound loaf to a 10,000 pound loaf. We’re making one loaf of bread over and over. The FDA recognizes that.
“The real challenge—a lot of what we have to do—is to educate the regulators about our particular manufacturing method,” Mayleben continued. That will be a challenge for the industry for the next decade, he predicted, until a body of knowledge accumulates.
Lessons Learned
TiGenix, which recently acquired Cellerix, is beginning Phase III trials with Cx601, according to Eduardo Bravo, CEO. Cx601 is based upon expanded adult stem cells derived from adipose tissue. These cells are being tested in osteoarthritis, rheumatoid arthritis, inflammation, and autoimmune diseases.
A previous Phase III trial with the autologous product Ontaril® targeted anal fistulas in non-Crohn disease patients and devised a robust protocol with the goal of quick commercialization. In discussing lessons learned, “There’s not much we would have done differently on that phase,” Bravo said. But, once results accrued, two areas required further attention in subsequent trials.
“We should have ensured a homogenous patient population,” Bravo said, and selected a population that was more severely ill. Because the population was not severely ill, “there was a much higher rate of spontaneous healing than was anticipated. If we were to do this trial again, we would ensure the patent population was better understood,” he said.
Continuing, Bravo emphasized that the application and treatment of Ontaril involves a surgical procedure. “We underestimated the fact that surgeons have their own ways of performing surgery,” he said. Although the variations in surgeon’s approaches and hospital protocols were not huge, he advised standardizing them for future trials.
TiGenix is applying those lessons to a Phase III trial currently being designed for complex anal fistulas in Crohn disease. “This is essentially the same disease, except there are more severe fistulas and more severe inflammation,” Bravo said. The patient population, therefore, is better understood and less likely to heal spontaneously.
Also, TiGenix has developed “very clear instructions on how surgery should be performed to ensure consistency and to avoid malfunction of the cells because of poor handling,” Bravo noted. Likewise, a Phase I/II trial for rheumatoid arthritis now includes one nurse to review administration and to ensure the proper manipulation of the cells.
Bravo said the commercialization pathway for Cx601 is not so different from that of other products. TiGenix is working closely with regulatory authorities and has gained approval of its manufacturing facilities. Based upon its previous work, “a single additional trial may be sufficient for registration,” Bravo said.
Strategy
Advanced Cell Technology (ACT) is enrolling patients who have either dry age-related macular degeneration (AMD) or Stargardt macular dystrophy in its Phase I retinal pigment epithelium trials. Preliminary results are expected by year’s end in the U.S. European trials are expected to begin the first half of 2012.
Targeting the orphan indication Stargardt macular dystrophy and the large-market dry AMD conditions creates a strategy that is expected to provide near-term funds and a long-term market potential, Gary Rabin, interim chairman and CEO, explained.
The firm uses single-blastomere technology, which generates human embryonic stem cells without destroying the embryos or changing their fate. The process uses a single-cell biopsy to extract a cell for a cell line that is five times more efficient than the best NIH lines for producing cells from all three germ layers, making it more reproducible and more robust than the traditional inner cell mass-derived lines, Rabin claims.
The embryonic stem cells are differentiated in vitro to fully differentiated (pigmented) RPE cells, which don’t need staining. They are administered in small doses, and minimal immunosuppression is needed.
After extraction, these cells are “coaxed to become RPE cells” and then administered to the patient through a subretinal injection—a procedure already used by eye surgeons. “In animal studies, we saw the complete arresting of disease, and sometimes some improvement,” Rabin said.
Fast Track
Osiris began its mesenchymal stem cell program in 1992 and won approval to begin Phase III trials in 2006. “We’re the senior citizen of stem cell companies,” Doug Jacobstein, M.D., senior director medical affairs, joked. Since then, Osiris has expanded its stem cell program to include anti-inflammatory applications.
“We have fast-track status for two indications: acute graft versus host disease (GvHD) and Crohn disease,” Dr. Jacobstein said. GvHD affects about half of all transplant patients, and there is no approved therapy. Standard treatment starts with high-dose steroids, which more than half of patients fail.
Protocol 280, a trial for steroid refractory GvHD, combined the physician’s choice of second-line therapy with either Prochymal® or a placebo. “Prochymal achieved an overall response in 82 percent of patients with gastrointestinal GvHD and in 76 percent of patients with GvHD of the liver,” Dr. Jacobstein reported.
Another trial, Protocol 275, involved 59 pediatric patients, of which 92% had visceral organ involvement, 88% had the most severe forms of GvHD (Grades C/D), and 63% had multiple organ GvHD. “At day 28, 63 percent had responded to Prochymal, with a median improvement of two grades.” Of patients who responded at 28 days after treatment, 78% were alive at day 100.
“Fast Track status didn’t affect the study design. The benefit is that it allows more frequent meetings and conversations with the FDA and allows a rolling review so the BLA—for example—may be filed in sections.” An NDA priority review under Fast Track takes about 6 months, versus about 10 months for nonpriority review.
Juventas Therapeutics gained FDA approval to initiate Phase II testing in critical limb ischemia using its lead compound, JVS-100, in January. Currently, there is no FDA-approved therapy, according to Rahul Aras, Ph.D., CEO.
JVS-100 encodes stromal-cell derived factor 1 (SDF-1). As Dr. Aras remarked, “SDF-1a is a very interesting molecule.” SDF-1 repairs the heart after myocardial infarction by activating the natural repair pathways, promoting the growth of new blood vessels, and preventing on-going cell death. Early studies indicate it may repair the heart when administered even a few months after a cardiac event.
“Results are promising,” he said. Specifically, “in the six minute walk, patients improved by 36 meters in four months and gained 22 points in quality-of-life measurements, and about half improved one full cardiac class.”
JVS-100 has the potential to be a platform drug, with applications after ischemia and traumatic injury to the brain, kidney, liver, lungs, and dermis. “We also have topical approaches to accelerate wound healing.”

Osiris’ technology is based on the work of Arnold Caplan and his colleagues at Case Western Reserve University, who showed that mesenchymal stem cells can engraft and selectively differentiate, based on the tissue environment, to such lineages as muscle, bone, cartilage, marrow stroma, tendon, and fat.