September 15, 2010 (Vol. 30, No. 16)
Lack of Standards across Most Aspects of Process Impedes Efforts to Improve Quality
The field of protein therapeutics has grown dramatically since the 1980s. Analytical developments along the way have opened the door to understanding protein chemistry and formulations. Current methods to improve the quality of protein formulation development were among the topics discussed at IBC’s recent “Formulation Strategies for Protein Therapeutics” meeting.
Freeze-drying does not fall under FDA or GMP guidelines; however, there are product stability guidelines. There are also some standards to follow when matching excipients with proteins to prevent protein degradation during freeze-drying. These standards are not foolproof though, and additional work or adjustments to optimize stability of the molecule are often necessary, explained Adora Padilla, Ph.D., scientist I at KBI Biopharma.
“Typically, it’s a matter of balancing the excipient with the molecule in order to optimize stability, while maintaining product quality. What we’re specifically trying to do is use excipients that are going to give an elegant cake—the appearance of a cake in the vial at the end of the process.”
A case study with a molecule highly susceptible to degradation during freeze-drying provided an interesting example. “The main problem was product collapse, which resulted in stability problems and degradation of the molecule upon processing the sample,” Dr. Padilla reported.
The product collapse was the result of an imbalance of excipients. “It’s important to understand what the state of the excipient is going to be during the process and the balance of crystal and amorphous excipients. Sometimes this has no implications on the stability of the molecule, but in this case it did result in degradation.”
Overall, the success of freeze-drying depends on the molecule. Some, like antibodies, are more stable in solution; other smaller proteins, such as enzymes, are often freeze-dried. Although there are some rules of thumb, Dr. Padilla thinks it’s not possible to standardize the process. “It’s something that has to be manipulated based on the actual protein.”

KBI Biopharma touts a broad range of product-development experience, with an emphasis on formulation, analytical development, process development, and clinical manufacturing.
Synthetic Agents
Seattle Genetics has developed a technology using synthetic agents called auristatins and linker systems that attach these auristatins to antibodies. The linkers are designed to be stable in the blood and release a potent cell-killing agent once inside the target cell. This approach was designed to reduce toxicity of traditional chemotherapy while enhancing antitumor activity.
Challenges in formulation development, said Shan Jiang, Ph.D., director of formulation, are due to the complex nature of antibody-drug conjugates (ADCs). Attaching various cytotoxins to the antibody can alter stability and solubility. It’s important to understand degradation pathways and evaluate physical and chemical instability of molecules.
“One needs to pay close attention to the quality attributes unique to ADCs such as drug-to-antibody molar ratio, sites of drug conjugation, conjugation drug-related impurities, and potency.”
The company has also developed various analytical methods to better develop ADCs. SGN-35 (brentuximab vedotin), one of its ADCs currently in clinical trials, has shown “encouraging activity in early-stage trials in Hodgkin lymphoma and anaplastic large cell lymphoma patients refractory to several forms of chemotherapy,” reported Peter Senter, Ph.D., vp, chemistry.
According to Dr. Senter, a specialized linker technology was developed for its auristatins, a class of antitubulin drugs that includes monomethyl auristatin E (MMAE) and monomethyl auristatin F (MMAF). “The linker incorporates conjugation technology that allows the drug to be attached to the antibody in a way that does not affect its pharmacokinetic properties.”
The combination of the drug, linker, and conjugation technology, along with the antibodies being used, has contributed to the positive results seen in the clinic to date, Dr. Senter said. He added that the technology is applicable to a wide variety of malignancies, and is also currently in trials for solid tumors with various antibodies.
“We found that the drug-linker technology we developed applies to almost all antibodies that we’ve looked at—and we’ve looked at hundreds.”
Although Seattle Genetics has studied its technology with artificial constructs called diabodies, Dr. Senter reported that they have no reason to believe those novel scaffolds will be better than antibodies for drug delivery. “Antibodies are ideally suited for what we’re doing because they stay in circulation a long time and are stable—they are close to ideal carriers for drugs.”
Enhancing Antibody-Drug Conjugates
There are three main challenges in developing antibody-drug conjugates, said John Lambert, Ph.D, executive vp of R&D and CSO at ImmunoGen. First, the antibody must be optimized for a given target. This takes into account how fast the antibody target is internalized and the route of internalization.
“Different antibodies binding to one target can influence the intracellular trafficking routes and perhaps the rate at which all those events occur.” Then the correct payload-linker combination must be selected.
“Different targets sufficiently traffic to lysosomes, where a noncleavable link would be appropriate. But, there are targets that do not traffic to lysosomes well and this would require a different linker.” A way to approach this is to make various compounds with different linker payload combinations and test them. The payload has to be potent. Two payloads that have yielded good activity on the tumor and are nontoxic and stable are maytansinoids and aurestatins.
“We think that each component is important and have learned a great deal about antigen selection and the nature of the antibody. One of the reasons we use a modular approach to our linkers is to allow for testing of different designs.”
The company’s approach to linker technology, called TAP, uses surface-accessible amino groups to avoid disturbing the structure of the antibody and, thus, yield stable compounds. Dr. Lambert explained that new linkers being developed include several with hydrophilic characteristics. When these are part of the linker, the payload linker lysine released in the cell can kill the cell, but also resists efflux pumps by multidrug resistance (MDR).
According to Dr. Lambert, these new linkers may allow for targeting of tumors that have a propensity to become resistant to chemotherapy.
Formulation challenges include distinguishing events that shorten shelf-life, which are antibody-specific versus those that are the properties of the ADC itself.
There are currently three different maytansinoid-linker compounds in clinical trials. “The exact property of a linker depends on the biology of the target and target cell. There isn’t a one-size fits all,” Dr. Lambert said.
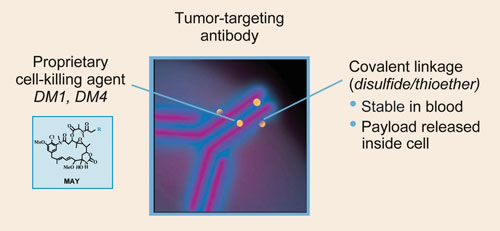
ImmunoGen says that its linker technology, TAP, arms antibodies with a potent cell-killing payload. Each TAP compound can then be tailored to optimize performance.
Stabilization of mAbs
Surface tension and conformational stability are closely related. The later refers to maintaining a protein in its native structure while in solution. “Something that is conformationaly unstable will tend to lose its native structure more easily and that will lead to problems—that’s true of antibodies and proteins of any type,” explained Tom Leach, Ph.D., scientist II, formulations, MedImmune.
Native structure is required for structure-specific function of proteins. For example, enzymes have an active site that requires certain native structure to catalyze a reaction. Antibodies must bind to an antigen—this is structure specific as well. Adding excipients and determining which ones are best for stability is a science that has been developed over the past few decades. There’s a limited number of excipients that are recognized as safe for pharmaceutical use.
Protein unfolding has been described as a struggle. On one hand, the protein wants to unfold and unravel in solution because it has thermal energy and wants to expand. On the other hand, the solution is exerting a surface tension that is trying to collapse the protein.
A protein trying to unfold is expanding against its own interface or a protein-water interface. “The interaction parameters are measuring the interaction of the excipients with the protein at this protein-water interface,” said Dr. Leach. Preferential exclusion indicates the excipient prefers to be excluded from that interface—an absence of interaction. Excipients that are preferentially excluded are usually the best stabilizers.
Some of the best-studied excipients are sugars, which tend to result in a more native protein structure. “If you add a little sugar to most proteins, you achieve some improvement of stability.”
One important measurement is the unfolding temperature—the temperature at which a protein in solution unfolds when under temperature stress. The addition of excipient adds protein stability over increased temperatures. “This can be somewhat dramatic. To improve stability over 10 degrees, one can substantially increase the shelf life for biopharmaceuticals,” Dr. Leach stated.
Aggregates and Subvisible Particles
Interest in protein aggregates was intensified when patients treated with therapeutic proteins became nonresponders. “Various researchers began to realize that maybe protein aggregates were the problem,” explained John Carpenter, Ph.D., professor of pharmaceutical biotechnology at the University of Colorado School of Pharmacy. “We realized the sweet spot for aggregates was in the subvisible range—probably around 10 microns.”
This is an important number, because all parenteral products have to be assayed for particles greater than 10 microns.
There is also interest in particles less than 10 microns as they may provide interesting quality product attributes that are not being analyzed nor reported in FDA filings. This has given rise to several issues: what is the smallest particle to worry about? is it a parameter that can be controlled? and are these particles an important issue for patient safety?
“The answer is, we’re not sure. If nothing else, it’s a product-quality issue and should be addressed.”
Although there is a lot of analytical work being done on protein particles, there is no definitive answer as to the best method. While particle counting is not difficult, it does have technical challenges that depend on factors like sample handling, and user experience.
Microflow imaging is becoming a popular method to study protein particles. In this technique, a microscope takes digital pictures of a field as fluid flows through it. It counts and averages the number of particles, which provides a distribution of the number of particles versus diameter.
A recent innovation by Graham Milne of Amgen uses video analysis on spun vials or syringes. The video of the particle behavior (sinking or floating), with their size and shape, enables noninvasive counting. This is helpful since all products require 100% visual inspection upon manufacture.
“This method may allow people to have quantitative data across the visible and subvisible, and may eliminate the arbitrary absolute that if you can see it, you can’t use it. By looking at the subvisible particles, companies gain valuable data that helps them to understand visible particles in the context of everything,” Dr. Carpenter noted.
Over the next year, there will be several in vitro and animal-based assays to analyze various aggregates and degraded proteins, he reported.
“In therapeutic proteins, we always worry about chemical modifications and particles are part of that.” However, he says, “it will be awhile before we really understand how to measure and control particles in products as a quality attribute. I don’t see anyone setting hard specifications for particles smaller than 10 microns for a while.”



