May 15, 2013 (Vol. 33, No. 10)
Twin-Column Ultra-High Resolution Chromatography
Chromatography is the most important unit operation in the downstream processing of biopharmaceuticals. In most cases, at least two chromatography steps are required to complete the purification: a so-called capture step and a polishing step. The purpose of the capture step is the concentration of the product and the removal of the largest part of the non-product-related impurities. The purpose of the polishing step is the removal of the remaining impurities, in particular of product-related impurities such as aggregates and fragments.
Traditionally, the capture and polishing chromatography steps are carried out in batch mode, having inherent drawbacks in achievable capacity, yield, and purity. Countercurrent chromatography is an ultra-high resolution chromatographic principle, which even when operated with only two columns can overcome these drawbacks to a large extent.
For capture steps, affinity chromatography is the method of choice. Since affinity chromatography is highly specific, it can purify the desired product with high yield. However, due to the elaborate and chemically susceptible ligands, affinity chromatography stationary phases are rather expensive. Protein A affinity chromatography is an exception as its capacity and chemical stability has been steadily optimized over the past decades, and commercial competition has lowered stationary phase prices.
Other affinity chromatography materials for newer applications, such as for the capture of mAb fragments, have not yet evolved this far and have significantly lower capacities and lower chemical resistance while being expensive.
Therefore, in particular for capture processes, it is of great importance to maximize stationary-phase capacity utilization in order to save cost and reduce processing time.
The dynamic binding capacity (DBC) is strongly dependent on the residence time of the product in the column. An increased feed flow rate increases the throughput of the process but decreases the residence time and thereby the breakthrough point, representing the point whereupon product is lost at continued loading. The breakthrough point is typically expressed as 1% DBC, which corresponds to the binding capacity achieved when the column is loaded until the breakthrough concentration exceeds 1% of the feed concentration.
In batch downstream processing, resin capacity is not used optimally as feed volumes of 80–90% of the 1% DBC value are loaded in order to avoid breakthrough.
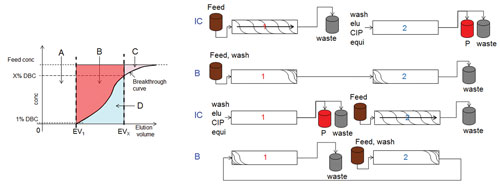
This context is explained in Figure 1. The area above the breakthrough curve (A+B+C) corresponds to the total mass of product that could be theoretically bound to the column (static binding capacity). The area A corresponds to the mass of product that is loaded at 1% DBC. Thus, the fraction of the capacity represented by the areas B and C is not utilized.
The additional capacity corresponding to the area B in Figure 1 is made available by sequential countercurrent-loading chromatography processes.
These cyclic processes feature two columns that are interconnected in one loading phase of the process (phase IC) and operated as batch columns for product elution and regeneration and continued loading (phase B). A process schematic of the twin column sequential loading process (CaptureSMB) is provided in Figure 1.
In the interconnected phases of the process, the columns are loaded in series with feed until a high X% DBC value for the upstream column is reached (X is typically 60–90%). According to Figure 1, the upstream column now contains the mass corresponding to A and B, while the mass corresponding to D has broken through into the second column where it has been re-adsorbed. The capacity utilization of the upstream column has dramatically improved without losing product. In the batch phase of the process, the upstream column is eluted, and the preloaded downstream column is continuing to be loaded.
A case study using Amsphere JWT 203 protein A stationary phase for the purification of an IgG1 from clarified cell culture harvest showed that for a similar productivity (14.0–16.5 g/L/h) and high recovery of 99%, the capacity utilization could be improved from 40% to 90%, corresponding to a load of 16.3 g/L (1% DBC) vs. 36.5 g/L (g mAb per L of column volume) when comparing the batch run to the twin-column sequential loading process. The mAb pool concentration increased from 3.3 g/L to 7.3 g/L, and the buffer consumption was decreased by 45% from 0.65 L/g to 0.29 L/g.
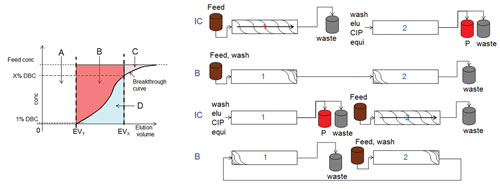
Figure 1. Schematic breakthrough curve in a chromatographic capture process (upper part), the additional capacity corresponding to the area B is utilized if a countercurrent sequential loading process is utilized. A schematic of a twin-column sequential loading process is shown in the lower part of the Figure.
Polishing
In difficult ternary separations such as in the case of a center-cut purification that removes early and late eluting product-related impurities, batch chromatography suffers from a trade-off between yield and purity.
Due to the overlap of the product peak and adjacent impurity peaks in the elution chromatogram, the impure side-fractions have to be discarded in order to obtain product within (purity) specifications, leading to substantial product losses (Figure 2). Pooling the impure side-fractions and the product increases the yield but lowers the purity. This situation represents the typical yield-purity-tradeoff of batch chromatography.
By means of multicolumn countercurrent solvent gradient purification chromatography (MCSGP), the yield-purity tradeoff can be defused. MCSGP uses twin columns and operates in a cyclic manner, whereby the product-containing side fractions are not discarded as in batch chromatography, but are kept in the system until the pure product has been extracted.
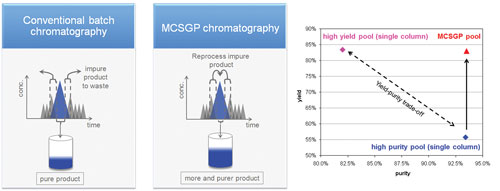
Figure 2. Principle of MCSGP chromatography for ternary separations (left parts). A process of this type was used for the purification of PEGylated a-Lactalbumin. Data obtained with single-column batch chromatography are shown for comparison.
In the case study presented in Figure 2, a twin-column MCSGP process was used for the purification of mono-PEGylated α-Lactalbumin from the native protein and higher order PEGamers, representing a challenging separation. Figure 2 shows that high yield and purity could be achieved simultaneously with the internal recycling of the product. The yield was increased from 56% to 83% for the purity of 93%. The productivity of the MCSGP process was twofold higher than the productivity of the batch run.
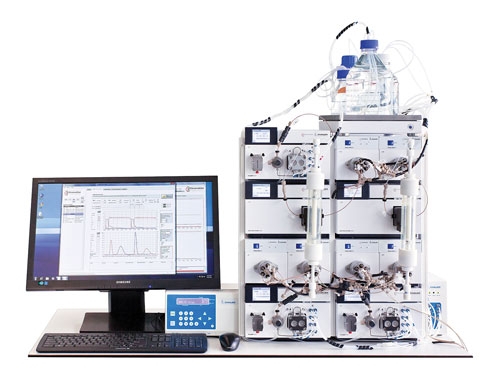
Figure 3. Contichrom Lab-10 equipment
Twin-Column Chromatography
The operation of twin-column sequential-loading capture processes (CaptureSMB) and counter-current polish purification processes (MCSGP) is facilitated by use of suitable hardware and software tools. The experimental data reported here was generated using the benchtop Contichrom Lab-10 equipment that is capable of running CaptureSMB, MCSGP, tandem processes, and single-column batch processes (Figure 3). The control software is particularly designed for developing and running cyclic chromatography processes.
Thomas Müller-Späth ([email protected]) is CSO, Guido Ströhlein is CEO, and Michael Bavand is CBO at ChromaCon.