February 15, 2010 (Vol. 30, No. 4)
Companies Invest in Array of Approaches to Accelerate Vaccine and Therapeutic Production
Since the H1N1 strain of influenza emerged last spring, there has been a flurry of activity to find strategies to treat the strain more effectively, to make doses of existing therapeutics go further, and to develop new vaccines more quickly than possible with the traditional chicken-and-egg method still used to produce most of the world’s vaccines. Some of those strategies are in Phase II trials now. Although they were not ready for the pandemic or for this year’s flu season, some of the most advanced options could be fast-tracked if the need becomes dire.
One of the production bottlenecks lies in applying the quality-by-design guidelines used successfully for small molecule drugs to biologics, according to Kevin Hrusovsky, president and CEO of Caliper Life Sciences. The challenge has been that cells behave differently in 50 L batches than in 500 L batches. Each change—in pressure, temperature, or oxygenation, for example—may affect efficacy. Therefore, thousands of tests are required. Capillary electrophoresis is too slow to provide the data the FDA needs in a realistic time frame, making quality by design virtually impossible for biologics.
Nicholas Moniotte, Ph.D., a scientist at GlaxoSmithKline Biologicals, has practical experience. His work concentrates on evaluating the performance of excipient molecules in formulation. “A good excipient must prevent antigen degradation during a stress/stability test. However, regulatory authorities ask pharmaceutical companies to implement quality-by-design approaches, particularly in the formulation-development process,” he explains.
“Such an approach requires the use of large condition screening and, thus, of high-throughput analytical technologies. Assessing the chemical integrity of an antigen at concentrations of approximately 100 µg/mL is a real challenge and is time-consuming,” using HPLC, isofocusing, capillary electrophoresis, and other process steps.
Hrusovsky says that Caliper’s LabChip GX overcame that challenge, providing high-throughput characterization of biologics and vaccines at a speed of up to 70 times faster than capillary electrophoresis. Consequently, tests that would have taken months can be completed in one day. “This is the first time you can get quality by design for biologics,” he notes.
Dr. Moniotte says he expects LabChip GXII use to expand at GSK from formulation to production applications because, as Hrusovsky adds, it has potential for in-line analytics. Its use can shave about one month off time-to-market for vaccines and other biologics. The LabChip GX also lets scientists perform the what-if tests that previously were impractical.
Hrusovsky, along with scientists from Pfizer, Amgen, and Biogen Idec, took that information to the FDA in November. The group presented to a standing-room only audience, describing their analytical approaches to biological characterization using the LabChip GX for high-throughput, predictive assessments of biological product quality. “The reviewers are comfortable with the technology,” Hrusovsky adds. “We are launching a pilot program with the FDA to make future BLA submissions” that adhere to quality-by-design principles using the LabChip GX.
Although process improvements can make significant contributions toward moving products through regulatory assessment and to the market more rapidly, most of the emphasis at Terrapinn’s recent “Influenza Congress” was on vaccines, Greg Went, CEO of Adamas Pharmaceuticals, reports. “This year’s pandemic strain will be next year’s seasonal flu. The flu is a recurring event that varies in severity from year to year, based mainly on how it shifts from previous seasons and the maturity of patients’ immune systems.”
In Went’s bottom-line assessment of the vaccine situation, “People are either at risk of complications from influenza or they’re not. Those with protective immune systems are primarily asymptomatic, regardless of whether they receive a flu vaccine.
“Those with unproductive, immunocompromised systems, even when vaccinated, don’t launch as robust an antibody response and so are more likely than others to contract influenza. Therefore, the goal of vaccines is to create a protective bubble around this immunocompromised group.” With pandemic vaccines in short supply, this bubble is less protective than desired.
That protective bubble can be enlarged by increasing supplies of vaccine. That, in turn, can be accomplished using new, faster production methods. Cell culturing is the first of those methods to approach commercialization in the U.S., evidenced by the opening of Novartis’ $1 billion cell culture facility in North Carolina in November. Novartis operates a similar facility in Germany to produce the H1N1 vaccine Celtura® and the seasonal flu vaccine Optaflu®.
When Novartis’ U.S. facility is fully licensed in 2013, it will be capable of producing 150 million doses of pandemic vaccine within six months, joining Sanofi Pasteur as the only vaccine developers with manufacturing facilities in the U.S. It could respond to a pandemic as early as 2011 if licensed in an emergency.
Rather than producing vaccine through cell culture, VaxInnate is growing the virus in E. coli. VaxInnate has been developing a vaccine for the Solomon Island strain of the H1N1 influenza since 2008. This approach can produce a vaccine in about six weeks—approximately 20 times faster than cell culture.
The company expects to begin Phase I trials in 2010 with 100 to 150 patients, followed by a 1,000-patient Phase II trial to determine immunogenicity. “By mid-2010, we’ll be in a position to take data to the FDA for emergency use,” according to Alan Shaw, Ph.D., president and CEO. “We’re about five years away from a standard submission, however.”
Another approach is to increase the concentrations of antigens in solution. NanoBio received a $9.3 million contract in October to further develop its nanoemulsion-based mucosal vaccine adjuvants for a variety of antigens. Data from different animal studies presented at that latest “ICAAC” conference indicated that the nanoemulsion is effective at 1/15th the standard antigen dose, without toxicity or tolerability concerns.
Phase I trials began last June. “We expect results in the spring,” says John Coffey, vp, business development. Although it’s too early to discuss outcomes, “we are seeing very good results.” If the vaccine offers similar protection in humans as in animal studies, it could become a flexible nasal vaccine that offers cross protection. It is being investigated for influenza, hepatitis B, Hib, RSV, cancer, anthrax, smallpox, and other diseases.
Novartis opened a $1 billion cell-culture facility in North Carolina in November.
Therapies
In terms of therapies, amantadine and oseltamivir are, so far, the most effective commercialized treatment options. Unfortunately, the Centers for Disease Control (CDC) has seen “a dozen or so Tamiflu-resistant viruses,” according to Anne Schuchat, director of the national center for immunization and respiratory diseases. The CDC is tracking those strains. Mid-December figures showed that 99.8% of the viruses tested were resistant to adamantanes, and that 0.8% were resistant to oseltamivir.
Adamas is addressing that threat with a triple-therapy combination of amantadine (an M2 inhibitor), ribavirin (a nucleoside analog), and oseltamivir (a neuraminidase inhibitor). In a paper presented at the “ICAAC” conference, researchers from Adamas, Utah State University, Amsterdam Medical Center, Naval Health Research Center, and the University of Alabama indicated that “the combination was highly synergistic in vitro, and the synergy was significantly greater than the synergy of double combinations against both sensitive and resistant viruses.” Specifically, the combination therapy was up to 20-fold more effective than monotherapy, and also was effective against oseltamivir- and amantadine-resistant strains. As yet, the reasons for this effectiveness remain unknown, although Went has some theories.
“Phase II trials are under way now,” he says. The drugs used in the combination therapy are FDA approved either alone or for other uses, so developing a new therapy should take only a few years rather than the 10–15 years typical of new drugs.
Researchers at the University of California, San Diego also are looking at existing drugs as treatment options, using rational drug design to identify fragments of FDA-approved medications to counter drug resistance in emerging viruses. The search targeted the neuraminidase proteins on the surface of influenza viruses, using an algorithm that also considers how the proteins shift positions and shapes over time.
So far, by mixing fragments of approved drugs, the McCammon lab has identified six compounds that may target neuraminidase more broadly and inhibit it better than the approved flu therapies oseltamivir, peramivir, and zanamivir.
One of the concerns regarding resistance is that if the virus resists Tamiflu, it may also resist the various vaccines. As yet, the CDC isn’t worried. “As our laboratory scientists look at hundreds of thousands of strains, we are not seeing changes that alter the vaccine protection. Still, even with these variations, the vaccines that have been developed are very good matches,” the CDC’s Dr. Schuchat says.
Despite 2009’s shortages of vaccines and the development of drug-resistant strains, the ability to respond to emerging pandemics is progressing steadily. The sense within the industry is that extraordinary measures like fast-tracking will be unnecessary. The next few years will see a dramatic improvement in the ability of manufacturers to produce effective vaccines and therapeutics quickly and robustly.
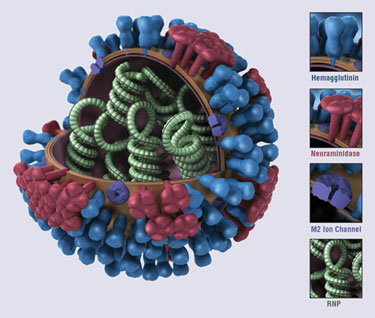
The biology and structure of a generic influenza virus (CDC)



