April 15, 2017 (Vol. 37, No. 8)
Consider Organs-On-Chips, Pathway Simulations, and Transgenic Mice
The road from a drug’s conception in the laboratory to its arrival in the pharmacy is long and arduous. It can also take over 12 years and cost over $350 million before a drug finds a place on pharmacy shelves.
The initial laboratory testing itself is an expensive, time-consuming task. The human body is a physiologically complex machine, and any introduction of new drug compound has the potential to cause multiple organ systems to malfunction and, in extreme cases, to shut down completely. If such adverse results could be prevented in the early stages, expensive road trips to nowhere could be cut short, or suitable detours could be mapped.
To help drug developers avoid bad road trips, cell biologists, safety pharmacologists, toxicologists, and other scientists will present some cutting-edge research at Predicting Drug Toxicity, a conference to be held June 13–14 in Boston. At this event, presenters and attendees will gather to discuss their common goal: minimizing drug-induced toxicity in new drug candidates. They will also reconsider the place of ADME/DMPK (absorption, distribution, metabolism, and excretion/drug metabolism, and pharmacokinetics) assessments in drug development.
DILISym
Heading up the big show in Boston as keynote speaker is Paul Watkins, M.D., director of the University of North Carolina Institute for Drug Safety Sciences and professor of medicine, pharmacy, and public health at the University of North Carolina, Chapel Hill. Dr. Watkins, an expert on drug-induced liver injury (DILI), has developed a computer model called DILISym.
The model can be used to assess the toxicity potential of drugs at various developmental stages before they reach clinical trials. It can also help developers explain and reduce toxicity that emerges in the clinic.
Systems with such capabilities are valuable in the drug development arena. What sets DILISym apart from other systems is that it has been developed with the guidance of many brilliant minds from most of the top 20 pharmaceutical companies, as well as the FDA. Keeping an open door for the latest developing drugs to run through the DILISym system allows pertinent information about liver safety to come to the forefront quickly.
“This model utilizes a ‘middle-out’ technique,” says Dr. Watkins. It starts with organ-level assessments of drug-induced liver injury, then delves more deeply, assessing cellular mechanisms, “when that is necessary to explain the liver injury.”
The DILISym model also has three submodels outlining the major pathways by which a drug may cause liver injury. These are via oxidative stress, interference with mitochondrial function, and alterations in bile acid homeostasis. Partner companies quantitatively test drugs on these pathways in the laboratory and directly plug the results into DILISym, thus expanding the database. The DILISym software can then be used to test drug compounds in various simulated populations, or “SimPops.”
Individuals within this database can express a wide range of responses to the compounds in question, allowing for population variability in genetic, disease, and environmental factors. DILISym has also been expanded to simulate liver damage in Beagle dogs, Sprague-Dawley rats, and C57BL/6 mice. It doesn’t hurt that DILISym modeling has already been presented in regulatory submissions including a recent NDA.
Body-on-a-Chip Devices
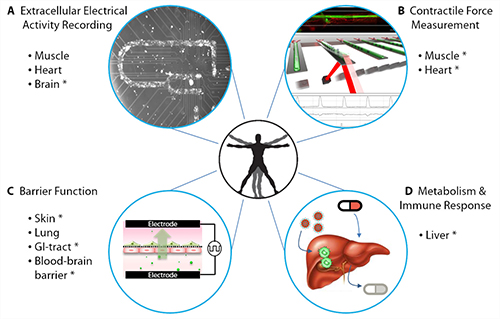
On the other end of the spectrum is a more personal approach. A collaboration between James Hickman, Ph.D., head of the Hybrid Systems Lab at the University of Central Florida, and Michael Shuler, Ph.D., professor of chemical and biomolecular engineering at Cornell University, focuses on the development of body-on-a-chip devices, microfluidic platforms that contain several compartments, each of which corresponds to a different organ. Each compartment contains cell cultures derived from the same individual.
What makes the body-on-a-chip device even more fascinating is its ability to circulate media between all the different compartments. The device uses a pumpless rocker platform, which induces a gravity flow that also keeps the shear stress on the cells within acceptable physiological ranges. By simulating circulation, the device allows for the evaluation of multiorgan toxicity in response to any potential developing drug.
“It is important to note that the medium is serum-free, cutting down on the variability that would normally be associated with having animal serum,” says Dr. Hickman. “This in turn improves upon the ability to predict drug efficacy with more accuracy.”
Drs. Hickman and Shuler have established a four-organ chip using cardiac, liver, skeletal, and neuronal cultures, and they have maintained these cultures over the course of 14 days in the aforementioned serum-free media. They are also working on expanding their platform, including maintaining 10 different organs on the same chip.
A device with such capabilities would enable groundbreaking advances in personalized healthcare. Cultures of cells from a diseased individual’s organs or derived from induced pluripotent stem cells could be maintained on the chip and treated with multiple drugs. Then, drugs identified as curative and nontoxic could be administered to the patient without fear of any adverse effects. The body-on-a-chip approach to drug evaluation could be even more valuable in treating patients who suffer from rare diseases.
Drs. Hickman and Shuler have founded a company called Hesperos that will specialize in developing body-on-a-chip technology for toxicological and efficacy testing. The company will also pursue other useful mechanistic assays for preclinical drug discovery.
The CiPA Initiative
Ventricular tachycardia due to torsades de pointes (TdP) has long been characterized and attributed to long QT intervals measured via electrocardiography. This phenomenon has been linked to the blockade of a potassium ion channel, a subunit of which is coded by the human ether-a-go-go-related gene (hERG).
Normally, this channel, called the rapidly activating delayed rectifier potassium channel (IKr), conducts a potassium current that plays a prominent role in defining repolarization of the ventricular action potential. So, it should occasion little surprise that many developing drugs have been taken off the drawing board simply because in vitro studies indicated that they had an ability to block the hERG channel.
However, not all drugs that block the hERG channel cause TdP, and an increase in the QT interval would not necessarily cause TdP, either. This is the basis of the Comprehensive in vitro Proarrhythmia Assay (CiPA) Initiative. Gary Gintant, Ph.D., senior research fellow at the department of integrative pharmacology and integrated science and technology at Abbvie, explains why it took so long to shift the focus off the hERG channel block and long QT syndrome being the gold standard for the diagnosis of TpD.
“Many factors slowed this shift,” Dr. Gintant explains. These include (a) “a generalized acceptance of the present approach by most (despite the possibility of removing drugs from drug development due to false-positive findings leading to discontinuation of novel compounds)” and (b) “changing the culture and structure built around hERG/QT testing. A true paradigm shift was achieved, he but it required the efforts of an enthusiastic group of followers (mostly volunteers) to raise awareness as well as generate data to convince tough-minded scientists. Such a group took time to build.”
The CiPA Initiative consists of three elements: 1) the assessment of drug effects on human ventricular ion channel currents, 2) in silico integration of these ion channel effects determining net effect on cardiac active potential, and 3) checking for discrepancies in fully integrated biological systems via stem cell-derived cardiac myocytes and human ECG.
Twenty-eight compounds have been selected for the study—12 for CiPA training/calibration, and 16 for validation. Before the compounds were included in the study, each of them was categorized according to its risk (high, intermediate, or low) of causing TdP.
The In Silico Working Group (ISWG) developed a computer model of the adult ventricular myocyte, which the CiPA Initiative uses to predict clinical risk of drug-induced TdP. Human cardiac stem cell derived cardiomyocytes (hSC-CMs) are then used to confirm the in silico results within the CiPA paradigm by assessing changes in the extracellular field potentials of spontaneously active hSC-CMs. This combination of both in silico and in vivo branches aims to diminish any doubts about the torsadogenic effects of drugs that have a positive benefit-to-risk ratio.
Animal Models
A huge challenge in predicting drug efficacy and toxicity is progressing from in vitro tests to animal models. Fortunately, animal models have shown continuous improvement. Contributing to this improvement is Taconic Biosciences, a provider of research models where Michael Seiler, Ph.D., works as a portfolio director. He focuses on commercializing genetically engineered models, such as Taconic’s rasH2 mouse model.
“The amazing thing about the rasH2 model, is that it cuts down the study time from two years to 26 weeks,” asserts Dr. Seiler. He adds that it not only saves time and money, but also allows for more accuracy by preventing spontaneous tumors and false positives.
This rapid tumorigenic response, which is achieved via the overexpression of a strong oncogene (ras-encoded p21 protein), gives the rasH2 model an advantage over many other transgenic models on the market. Aging effects are also minimized. The rasH2 model is also useful in evaluating tumor treatment compounds.
Drug toxicity is the major reason for most withdrawal of approved drugs from the market, the most common reasons being hepatotoxicity and cardiotoxicty. Usually, toxicity testing is completed and results are vetted in the preclinical phase. But even if drugs pass through these evaluations with high marks, they may falter after they enter the clinical market. Here, toxicity reports may reflect adverse consequences among end consumers.
Such revelations may trigger losses that far exceed those associated with a drug’s research and development expenditures. A drug that hurts consumers can lead to litigation and reputational damage. Drug debacles, however, can become less common. For example, if innovations such as the DILISym system, body-on-a-chip technology, or tests consonant with the CiPA Initiative are used to improve toxicity testing in the early stages of drug discovery, many problems down the line can be eliminated.
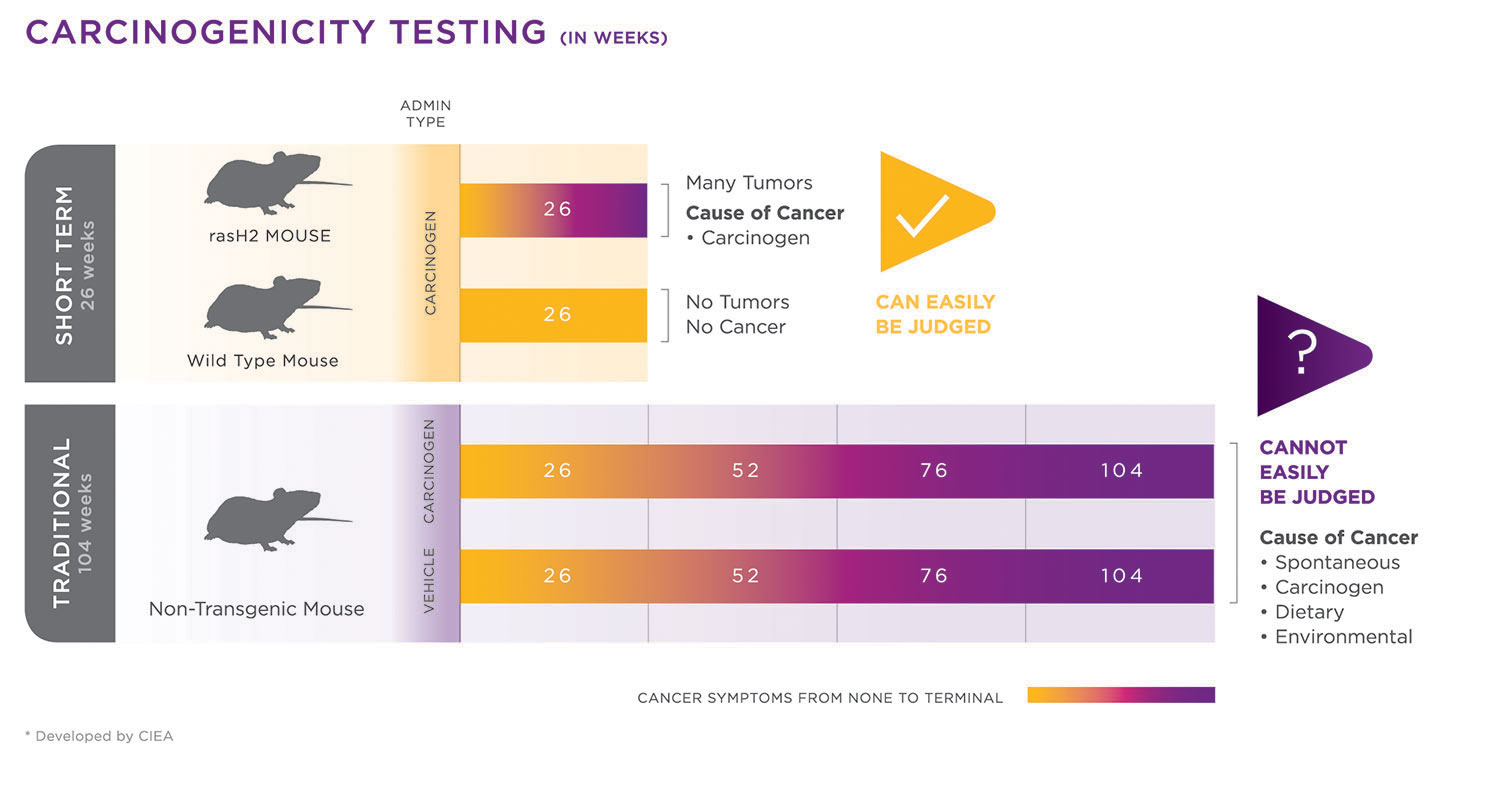
A comparison of carcinogenicity study timelines for Taconic Biosciences’ rasH2 model and a traditional mouse model. The faster tumorigenic response in the rasH2 model allows studies to be completed relatively quickly (in about six months instead of two years). In addition, since a higher proportion of mice will respond to carcinogen exposure, fewer animals are required for study completion compared to lifetime rodent assays. [Developed by CIEA]
De-Risking Drug Development Using Nonanimal Technologies
Given the global demand for safe and efficacious new medicines to treat cancer, metabolic disorders, mental illness, and other diseases, the biopharmaceutical industry drug pipeline continues to grow. However, drug development is an expensive, long process involving high rates of new molecule attrition. It is imperative, therefore, to be able to advance new compounds that have a lower risk of failure. This is the essence of de-risking—the intelligent application of relevant early testing to help mitigate major drug development risks such as toxicity, complex clinical development, nonapproval, and postmarket withdrawal.
Key areas of focus for early de-risking include the susceptibility a new drug may have to suffer from drug-drug interactions (DDIs), genotoxicity, cardiotoxicity, neurotoxicity, and drug-induced liver injury (DILI). If liability in any of these major risk areas is detected early enough, it is possible to make changes to the drug’s structural chemistry. Such changes should retain the drug’s pharmacophore but reduce its safety liability.
Over the past decade, in vitro models have become increasingly sophisticated with primary human cell function and differentiation being maintained for several weeks. Indeed, integrated culture systems that incorporate multiple primary cell types have led to the concept of “humans on a chip.” Such chips can allow efficacy and safety risks to be assessed on multiple human cell types. Other new in vitro technologies include 3D bioprinting, genetic modification of primary human cells to evoke proliferation, co-culturing, and hanging-drop technology (liquid surface tension) to enhance 3D cell culture.
All of these new in vitro technologies play a crucial role in advancing de-risking strategies using human cells. The primary aim of Envigo’s de-risking strategy is to provide a comprehensive and rigorous data set in early drug development that facilitates rational, scientifically based assessments to identify particular characteristics of drugs that result in failure.