June 15, 2010 (Vol. 30, No. 12)
Answers Likely to Be Found in Unraveling Genomic and Epigenomic Complexities
At present it is estimated that only about 15 out of 100 women with breast cancer treated with chemotherapy achieve any benefit. Current cancer treatments have limited efficacy, are toxic, and are typically quite costly.
The monoclonal antibody-based drug cetuximab, which is used to treat colorectal cancer, for example, costs about $12,000 for a four-week treatment. The goal of personalized medicine is to identify novel therapies that more effectively target a well-defined population of tumor cells and to pinpoint the patients in whom these treatments will be more likely to produce positive outcomes, thereby improving efficacy, limiting toxicity, and reducing treatment costs. Although no one drug is likely to stop cancer, the hope is that combinations of compounds that target multiple sites in a relevant biochemical pathway or signaling cascade will be efficacious.
Arul Chinnaiyan, M.D., Ph.D., professor of pathology and urology at the University of Michigan Medical School, chaired a plenary session on the complexity of the cancer genome at the American Association of Cancer Researchers (AACR) annual meeting held recently in Washington, D.C.
Dr. Chinnaiyan spoke of the need for comprehensive analysis to dissect molecular differences between tumor and normal cells and between different tumor subtypes. Enabling technologies such as amplification techniques, high-throughput parallel sequencing, and, more recently, single molecule sequencing are making this possible. Now the focus is moving to the transcriptome and the identification of chimeric RNAs, he told AACR attendees, as well as to epigenetics and understanding the methylation states of normal versus cancer genomes.
Illustrating the complexity in trying to characterize the cancer genome, Dr. Chinnaiyan explained that some mutations can cross tumor types whereas others will not be shared by tumors of the same type. He pointed to the challenges in trying to connect the dots between the genome and the epigenome and to define the mechanisms by which epigenomic alterations, such as changes affecting histones and chromosomal structure, can affect gene expression.
How can you personalize cancer therapy when many driver mutations may be present in 1% or fewer tumors, and how can you use this information to design clinical trials? he asked. In addition, he wondered, “Should we begin to increase the importance of driver mutations versus tissue of origin for classifying cancer?”

Many cancer researchers maintain that the successful development of personalized medicine will largely depend on the merging of clinical and molecular data. [Corbis/Fotolia]
Coping with Complexity
Bert Vogelstein, M.D., Clayton professor of oncology and pathology at Johns Hopkins University, summarized the information collected to date from the first 100 cancer genomes that have been analyzed, 78 of which are published. Across various tumor types, the number of genetic alterations—primarily point mutations—typically ranges from 30 to 80. In pancreatic cancer, for example, the median number of altered genes is 44, with a range of 36 to 60.
For lung and melanoma tumors, the number of mutations increases to 100–200, which can be explained by the addition of a variety of alterations caused by carcinogens associated with tobacco and sun exposure. Leukemia and medulloblastoma, which develop more quickly, typically contain about 10 cancer-associated mutations.
The data indicates that mutations tend to accumulate as a tumor ages and evolves, and that most alterations are “passenger” mutations, rather than “driver” mutations, the latter of which are present in tumor suppressor genes or oncogenes and are directly responsible for driving oncogenic transformation. Differentiating driver from passenger mutations is a key challenge and the focus of much current research.
Of the 3,142 mutated (and potential) cancer genes found in these 100 genomes, about 286 are in tumor suppressor genes. This has important implications for drug discovery, noted Dr. Vogelstein, as “you can’t target something that is being suppressed.” Nearly all of the driver genes are part of 12 core signaling pathways.
Dr. Vogelstein described tumor heterogeneity as the “elephant in the room.” For example, two different people may have the same type of tumor with mutations affecting the same signaling pathway, but the mutations may be in different genes.
Most cancer genome studies to date have been done in model organisms, and Dr. Vogelstein emphasized the need to perform large-scale genome analysis studies in human cancer cells and the importance of developing an understanding of the pathways through which cancer genes operate in human cancer cells. This information will be essential for translating genomic information to drug discovery and for targeting pathways such as DNA repair pathways, angiogenesis pathways, and metabolic pathways—rather than individual mutant proteins.
Over the past 30 years, scientists have come to understand “most of the genes and all of the pathways in cancer,” said Dr. Vogelstein, and he does not expect any surprises such as the discovery of new cancer genes. “We know the landscape,” and it is sobering, he said, noting the complexity and challenges the cancer genome presents. Yet he is optimistic that “the mitigation of much disease caused by cancer is within our grasp.”
Translational Genomics
The development of personalized medicine will depend on the merging of clinical and molecular data, according to Laura van ’t Veer, Ph.D., head of molecular pathology at The Netherlands Cancer Institute, who is studying gene-expression profiles in women with breast cancer and discovering how this information can be used to guide treatment decisions and predict prognoses.
Dr. van ’t Veer described a 6,000-patient trial being conducted in 21 countries in which the MammaPrint Profile, a 70-gene prognostic signature from Agendia, is being used as a prognostic tool to predict cancer recurrence risk and to identify patients with early-stage breast cancer who are likely to benefit from adjuvant chemotherapy. The protocol includes banking of FFPE and frozen tumor tissue as well as blood for analysis.
Described by the study investigators, from the University of Texas M.D. Anderson Cancer Center, as “the first completed biopsy-mandated study in pretreated NSCLC,” the BATTLE trial assessed disease control after eight weeks of drug treatment. Results of the Phase II trial were revealed at the AACR meeting.
Biopsy specimens from 255 patients with non-small-cell lung cancer (NSCLC) were analyzed for 11 biomarkers from four molecular pathways implicated in NSCLC, and this information was used in the second phase of the trial to randomize patients into four drug treatment groups. Each of the drugs targets one of these four pathways. In the first phase of the trial, patients were equally randomized to receive any one of the four study drugs (or drug combinations), whereas in the second phase, patients were adaptively randomized for drug treatment based on their biomarker profiles.
The results supported the pretrial hypotheses. Among patients with a mutation in the KRAS pathway, for example, 61% of those treated with the drug sorafenib, which targets the KRAS pathway, exhibited disease control at eight weeks, compared to 32% of those who received one of the other three drugs. Overall, 46% of the patients in the trial had disease control at eight weeks, compared to the historical experience of about 30% among patients with late-stage lung cancer.
Combination PET/CT image of a mouse with two HeLa tumor xenografts implanted, one on each leg, after radiation therapy: Metabolism within the tumors as imaged by 18F-FDG uptake into the tumor sites post treatment with radiation therapy. The top tumor was irradiated with 25 Gy and the bottomtumor with 10 Gy. Tumors exhibited the expected decrease in uptake that correlated with increasing treatment dose.[Carestream Molecular Imaging]
Targeting the Cancer Genome
Levi Garraway, M.D., Ph.D., assistant professor of medicine at Dana-Farber Cancer Institute, presented information from his group’s work to characterize the genomic alterations in melanoma. Statistically significant amplifications, deletions, and mutations identified from the study of 100 genomes are likely to be nonrandom, Dr. Garraway told the attendees, and several have been described in recognized oncogenes and tumor suppressor genes associated with melanoma. These include amplification of BRAF and MITF and alterations in the ETV1 transcription factor.
Knockdown of ETV1 has been shown to reduce melanoma cell proliferation, and ETV1 expression has been associated with substantial accumulation of the MITF oncogene. In the setting of MAP kinase activation, the addition of ETV1 can promote melanocyte transformation.
Dr. Garraway described ongoing research to identify small molecules that can target this signaling pathway at multiple levels. This work involves the use of microarrays to identify ligands that bind target transcription factors, and the use of SPR technology to measure small molecule/target protein binding events. The results suggest that ligand binding can reduce ETV1 expression. Dr. Garraway envisions the development of a “multipronged approach to attack a pathway and intercept it fully.”
Massively parallel next-generation sequencing is enabling whole-genome analysis and is replacing capillary- and array-based technologies, noted Dr. Garraway. It is helping researchers “characterize the RNA world in melanoma” and identify chromosomal rearrangements and novel fusion genes. Pilot studies have demonstrated the presence of highly chimeric transcripts and evidence of alternative splicing in fusion genes.
“Most gene fusions we see in melanoma are not recurrent,” said Dr. Garraway, emphasizing the importance of distinguishing between the genomic alterations that drive oncogenic transformation versus those that are merely passengers, or perhaps even “chauffeurs” or “alternative drivers,” which he described as alterations present in a particular tumor that cannot be generalized across tumors of that type.
Dr. Garraway underscored the need to weave together genetic and functional information, combining the results of cancer genome analysis with functional/phenotypic alterations identified in tumor cells using techniques such as pooled RNAi screening.
Ben Ho Park, M.D., Ph.D., associate professor of oncology at Johns Hopkins Hospital, described the development of models of somatic cell knock-ins of mutant PIK3CA at the meeting. PIK encodes the catalytic component of P13 kinase, and “hotspot mutations account for most alterations.” The P13 kinase pathway is involved in downstream AKT phosphorylation events.
To study these mutations and begin to search for targeted therapeutic interventions, Dr. Park’s group created somatic knock-in cell lines, in which they introduced the mutant form of the gene, yielding 50–50 expression of the mutant and normal gene, rather than overexpressing the gene of interest. Studies of these oncogenic PIK3CA mutant knock-in models have led to the identification of downstream signaling pathways that might contain targets for therapeutic intervention.
These model systems can be used to screen compound libraries to potential drug candidates. Dr. Park presented data showing that lithium, for example, selectively targets mutant PIK3CA knock-in cells.
Alex Adjei, M.D., Ph.D., senior vp of clinical research at Roswell Park Cancer Center, presented study data for a small molecule inhibitor of a mutant BRAF gene found in melanoma. The treatment led to 85% tumor shrinkage in visceral lesions that contained the BRAF mutation and produced no benefit in patients with tumors expressing wild-type BRAF.
Dr. Adjei provided other examples in which agents designed to target driver genes are generating positive responses. While these types of results are promising and demonstrate the ability to target specific genes and mutations, he echoed the views of the other speakers, highlighting the need to broaden this approach and target multiple points along disease-associated pathway. “Pathway redundancy” in therapeutic interventions will be crucial, he noted, as tumor cells are resilient, and when one pathway is blocked, they will use another.
IDT scientists perform mass spectrometry QC on oligonucleotides at the company’s Coralville, Iowa, main headquarters.
Sidebar: AACR New Product Report
> Life Technologies introduced the Applied Biosystems ViiA™ 7 RT-PCR system, which integrates quantitative PCR and genotyping applications on one platform. The system’s software can integrate real-time PCR applications such as nucleic acid quantification, SNP detection, and copy number variation analysis with applications such as high-resolution melting, proximity ligation technology for protein analysis, and gene expression without RNA purification.
Within the ViiA 7, the OptiFlex system allows users to detect up to 21 colors to enable multiplexing, and to run TaqMan and SYBR-based assays in the same run. Company officials said it also provides single-copy detection sensitivity.
> 454 Life Sciences, a Roche company, previewed the GS Junior sequencing system, set to launch this month. The GS Junior incorporates the long-read GS FLX Titanium chemistry of the Genome Sequencer FLX in a benchtop, lower-cost format.
The company also announced upcoming improvements to its Genome Sequencer FLX, including enhanced software, microfluidics, library construction, and sample preparation, that will reportedly allow for read lengths of up to 1,000 base pairs.
> Scientists at Solulink said they developed a crosslinking chemistry capable of linking all types of molecules, which retain their functionality when combined. The company’s Antibody-Oligonucleotide All-in-One conjugation kit links oligos of 20–60 base pairs in length to an antibody, creating an entity with dual functionality, according to the firm.
The technology can also be used to immobilize molecules on the surface of arrays, to link enzymes to other types of molecules, or to do dye labeling. The controlled linking process allows molecules to be joined in any desired ratio. The linker has a signature wavelength.
Solulink products include ChromaLink™ biotinylation kits, crosslinking and antibody labeling kits, and NanoLink™ and MagnaLink™ strepatvidin-coated magnetic beads in 0.8 µm and 2.8 µm sizes.
> NanoString Technologies unveiled the nCounter® miRNA expression assay kits at AACR, which are capable of highly multiplexed, direct digital detection and counting of miRNAs in a single reaction without amplification, according to the company. The kits are compatible with the nCounter analysis system workflow. The nCounter Human miRNA expression assay kit contains 736 human and human-associated viral miRNAs, which are ligated unique oligonucleotide tags to enable selective and specific detection, added a NanoString official.
> GE Life Sciences presented the NanoVue Plus UV/visible spectrophotometer with predefined methods for quantifying nucleic acids and proteins. It provides measurements in sample volumes as small as 0.5 µL to 5 µL and utilizes a sample plate that eliminates the need for cuvettes or capillaries.
The company also showcased its Mag Sepharose™ platform of magnetic Sepharose beads. The platform uses Protein A, Protein G, or NHS ligands to immunoprecipitate target proteins, or TiO2 to capture phosphorylated peptides from digested protein samples.
> Thermo Fisher Scientific featured its Solaris™ qPCR gene-expression assays and highlighted their applications in cancer research, such as the ability to use qPCR to detect members of the AKT protein kinase family in an RNAi-based study of FOX01 regulation.
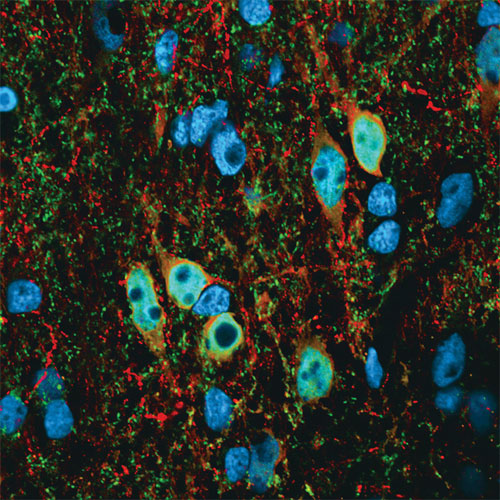
ChromaLink Biotin and Solulink’s antibody labeling kits allow for multicolor immunofluorescence (IF) labeling of cells using primary antibodies raised in the same host species. All Solulink antibody labeling kits include purification columns for producing a 100% pure conjugate, useful for applications such as IF where high-purity conjugates are necessary.
> As part of its PrimeTime® qPCR product line, Integrated DNA Technologies (IDT) developed the ZEN™ internal quencher, which allows for the production of double-quenched probes with a distance of nine basepairs between the dye and the quencher.
> PhosphoScout® technology from Kinaxo Biotechnologies is a quantitative phosphoproteomics platform that relies on quantitative LC-MS/MS to identify regulated phosphorylation sites across the proteome. It can monitor up to 15,000 in vivo phosphorylation sites in one experiment and compare phosphorylation patterns between samples.
> PerkinElmer’s Lance® line of TR-FRET, homogeneous, antibody-based assays now covers more than 50 kinases and includes the new Lance cAMP and Ultra cAMP assays.
> In Q3, RainDance Technologies plans to launch a new PCR amplification method for differentiating methylated DNA sequences.
> Carestream Molecular Imaging presented its Albira modular PET/SPECT/CT imaging system. Customers can purchase the complete trimodal system, a bimodal PET/CT or SPECT/CT, or a 1, 2-, or 3-ring PET system. The platform’s PET detection technology is based on a continuous crystal, rather than crystal grids, eliminating dead zones, said a company spokesperson, who added that the product is also able to correct for parallax error. The system’s software and electronics enable multimodal image acquisition and reconstruction to facilitate the study of biological processes quantitatively at the cell or tissue level.

IDT scientists perform mass spectrometry QC on oligonucleotides at the company’s Coralville, Iowa, main headquarters.
> Caliper Life Sciences launched the Quantum FX micro-CT system, which has an 18 second scan time for low-dose imaging; it allows for serial CT studies in a single animal to support longitudinal preclinical studies.
> Maestro™ is an optical in vivo imaging system from Cambridge Research & Instrumentation that was designed to distinguish and quantify multiple fluorophores emitting from the green to the near-infrared spectrum and differentiate signal from autofluorescence. Intended for small animal imaging, the system can acquire a full multispectral image in 5 to 10 seconds or 10 frames per second for a monochrome image, according to the company. The Maestro line includes the new Dynamic Contrast Enhancement (DyCE™) system and the new Maestro EX.
> In a poster presentation, the use of Sigma-Aldrich’s zinc finger nuclease (ZFN) technology to create rat models of disease for cancer research was described at the AACR meeting. The company’s CompoZr® ZFN technology relies on a ZFN pair that combines targeted DNA binding and DNA cleaving capability, creating double-stranded breaks in DNA at the targeted site. Sigma introduced its new p53 knockout rat model, which is being developed through Sigma Advanced Genetic Engineering (SAGE™) Labs.
> BioCytics’ human application laboratory offers a circulating tumor cell (CTC) enumeration and analysis service that utilizes the CellSearch® CTC test from Veridex to capture and assess tumor cells in the bloodstream. The test is FDA-approved for monitoring metastatic breast, prostate, and colorectal cancers.
BioCytics uses fluorescent immunostaining protocols to identify prognostic biomarkers on CTCs to help guide treatment decisions. The company isolates, banks, and looks for markers on CTCs, and is developing protocols for genetic analysis of CTCs.
> The Scepter cytometer from Millipore is a handheld automated cell counter that displays cell counts and population distribution histograms on a screen built into the device. On average, Scepter generates data in less than 20 seconds.
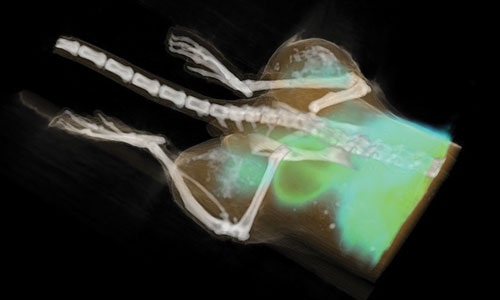
Combination PET/CT image of a mouse with two HeLa tumor xenografts implanted, one on each leg, after radiation therapy: Metabolism within the tumors as imaged by 18F-FDG uptake into the tumor sites post treatment with radiation therapy. The top tumor was irradiated with 25 Gy and the bottom tumor with 10 Gy. Tumors exhibited the expected decrease in uptake that correlated with increasing treatment dose. [Carestream Molecular Imaging/Drs. Medina and Garcia-Lopez, INCan-UNAM]



