December 1, 2013 (Vol. 33, No. 21)
Common Mistakes to Avoid in Order to Improve Translation of Interaction Data to the Clinic
Since 2006, when the U.S. FDA published a draft guidance on drug interaction studies, it has expected new drug applications to include in vitro data on the interactions of new drug candidates with drug transporters. The rationale is that if a new drug is a substrate of a transporter (e.g., an efflux transporter such as P-glycoprotein [P gp/MDR1]), its absorption, metabolism, distribution, or elimination could be affected by a co-medication that is a substrate or inhibitor of the same transporter.
Conversely, if a new drug is an inhibitor of a transporter (e.g., an uptake transporter such as OATP1B1), it could alter the disposition of a co-dosed drug (e.g., a statin) that is a substrate of the same transporter.
In either case, the consequences could be loss of efficacy and/or compromised safety of the victim drug. Not only is in vitro drug-transporter interaction data “expected” (in fact, review of some NDAs has been delayed until such data is included), but an implicit assumption is that they should be performed in such a way that the results predict what will happen in patients. Surprisingly, this is not always as easy as it sounds.
This article will cover some of the common mistakes to avoid in order to improve the translation of in vitro transporter interaction data to the clinic.
Drug developers are certainly complying with the FDA’s expectations; of the 24 oral or IV small molecule drugs approved by the agency in 2012, 18 have labels that reference transporters. Not only that, fewer and fewer labels reference clinical transporter data because the data is being generated in vitro instead. It seems clear that the FDA is pushing so hard to get in vitro transporter data in order to fill in the current gap in our knowledge about the clinical importance of transporter-mediated drug-drug interactions (DDIs) and the translatability of in vitro test systems.
Within a few years, one hopes that they will have accumulated sufficient data to determine whether the intelligent use of vitro transporter assays can make drug development both faster and safer when it comes to investigating the potential for clinical DDIs.
In the meantime, it is imperative to take into account a few important considerations that can mean the difference between a true result and a false negative (drug regulators have extremely low tolerance for these) or false positive (which will eventually be sorted out later, in clinical trials if necessary). These include:
- Properties of the test compound
- The optimal test system (depending on, e.g., the properties of the test compound)
- Test conditions
Test Compound
Do you have the concentration(s) of the test compound you think you have in your in vitro assay? After carefully digesting the relevant regulatory guidance documents, you know the relevant concentration range; for transporter inhibition, it is typically a multiple of the plasma Cmax (total or unbound, depending on the agency) at the highest proposed clinical dose or a fraction of the estimated concentration in the lumen of the small intestine (highest dose divided by 250 mL). So you prepare a dosing solution at the proper concentration. But what concentration of test compound is available to interact with the test system? Don’t assume anything until you have tested:
- Solubility—this can often limit the test concentrations that can be achieved in vitro, sometimes preventing you from reaching the target concentration for inhibition specified in regulatory guidelines (especially for transporters active in the gut such as P-gp and BCRP). The result can be understimation of inhibitory potency and overestimation of substrate potential.
- Nonspecific binding—relatively large, nonpolar compounds tend to be very sticky, binding nonspecifically not only to the plastic plates in which assays are performed but also pipette tips, cell membranes, etc. As a result, the actual concentration in solution can be much lower than expected, exerting a negative bias on (i) the measured rate of transport of the test compound (potentially leading to false negative transporter substrate assessment) and (ii) inhibition of transport of a probe substrate (potentially leading to false negative inhibitor assessment). This is illustrated in Figure 1.
- Chemical stability—As with the other two factors just cited, if a compound is not stable under the conditions of the test its actual concentration will be less than the nominal concentration, resulting in a negative bias.
- Tolerability—there is a risk of false negatives or false positives if tolerability of the cells to the highest concentration of the test compound is not confirmed.
These considerations collectively comprise suitability testing and should be looked at before a transporter assay is run, to ensure the validity of the results of the assay.
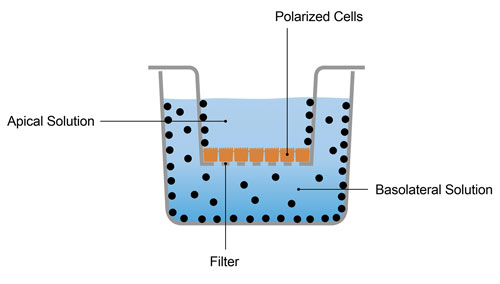
Figure 1. Some compounds (represented by black dots, which were added to the basolateral compartment) bind nonspecifically to plastic, which can greatly reduce the concentration in solution, especially in the receiver compartment. One might falsely conclude from the experiment illustrated here that the compound is not transported in the basolateral- to-apical direction.
Test System
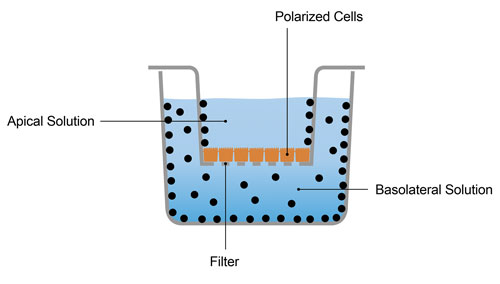
Efflux transporters such as P-gp, BCRP, and BSEP are tested with one of two assay formats, (i) bidirectional transport across monolayers of a polarized epithelial cell line (e.g., Caco-2 or transfected MDCK cells) in a dual-chamber plate, or (ii) uptake into inverted plasma membrane vesicles prepared from cells expressing the transporter of interest. Either false negatives or false positives can result unless the characteristics of the test compound are matched to the optimal test system.
Transporter-expressing inverted vesicles fail to identify (false negative) transporter substrates with high passive permeability because the compounds leak back out of the vesicles almost as fast as they are pumped in (Figure 2). Cell lines such as Caco-2, with native expression of P-gp and BCRP, can also fail with highly permeable compounds when active efflux (greater in the basolateral-to-apical direction than apical-to-basolateral) is small in magnitude compared to passive permeability (which is the same in both directions).
For these reasons, the only test system that may be able to identify this type of substrate is a bidirectional assay with a cell line such as MDR1-MDCK, in which P-gp is over-expressed. It should be noted that regulatory agencies are less concerned with in vitro assays missing highly permeable substrates of an efflux transporter because such compounds are unlikely to be victims of DDIs involving the transporter.
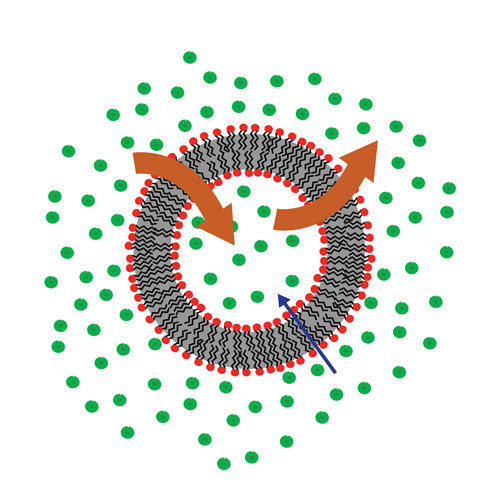
Figure 2. Inverted vesicles actively take up efflux substrates (represented by the inwardly directed blue arrow). However, they can result in false negatives for transporter substrates with high passive permeability (large brown arrows pointing in both directions).
On the other hand, for compounds with low to moderate intrinsic permeability it is important to accurately identify transporters for which they are substrates, mainly because of the risk of clinically significant DDIs. For this class of compounds, either inverted vesicles or natively expressing cell lines can be used but the latter may be more clinically relevant due to expression of the full (or nearly full) complement of both uptake and efflux transporters that a drug will encounter in the body.
The importance of the interplay between multiple transporters, including an uptake transporter on one end of a polarized cell and an efflux transporter on the other, is probably under-appreciated. It is noteworthy that overexpressing cell lines such as MDR1-MDCK can result in either false negatives (for compounds requiring a basolateral uptake transporter) or false positives (exaggeration of a minor substrate interaction due to non-physiological expression levels of the efflux transporter) with this type of compound.
To complicate matters even further, the European Medicines Agency (EMA) expects sponsors to evaluate drug candidates as substrates of efflux transporters in two different in vitro test systems, one (e.g., Caco-2) to test for DDI risk involving intestinal absorption and another (e.g., MDR1-MDCK) for DDIs involving systemic disposition.
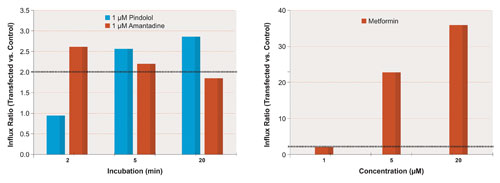
Figure 3. Pindolol, amantadine, and metformin are all substrates of the renal uptake transporter OCT2 (influx ratio >2 under at least one set of in vitro assay conditions); however, one could easily obtain a false-negative result by arbitrarily selecting a single set of conditions for screening. This can be avoided by a test matrix of several time points (left-hand panel) and concentrations (right-hand panel).
Test Conditions
At least when evaluating a compound as a substrate of uptake transporters, it is a good idea to test a matrix consisting of several test concentrations and several time points; otherwise, there is risk of false negatives as shown in Figure 3.
Chris Bode, Ph.D. ([email protected]), is VP of scientific and corporate communications at Absorption Systems.