February 1, 2010 (Vol. 30, No. 3)
Multiplex Gene-Expression Assay for Human Induced Pluripotent Stem Cells
Recent methods to reprogram human somatic cells in order to create induced pluripotent stem cells (iPSCs) are intended to provide important tools for drug discovery and models for the study of disease. The ultimate goal is to create an alternative to controversial embryonic stem cells (ESCs) and eventually generate patient-specific pluripotent cells for autologous cell therapy.
Reprogramming methods include the transfer of genetic material, protein transduction and/or application of chemicals to promote epigenetic modification, and the expression of key pluripotency markers. The reprogramming process involves repression of differentiation-associated genes, upregulation of endogenous pluripotency-associated genes, and, in the case of retroviral transduction, natural silencing of exogenous reprogramming factors.
Current reprogramming methodology is reproducible, and a single induction experiment may generate several candidate iPSC clones. However, only a handful of derivative cell lines attain a fully reprogrammed state and are suitable for subsequent downstream applications. A technical challenge faced by researchers is the ability to rapidly and accurately identify the iPSC lines that most resemble ESCs.
A multiplex reverse transcription polymerase chain reaction (RT-PCR) method surmounts this challenge by offering simultaneous, quantitative detection of gene expression for up to 30 genes with a minimal requirement for template RNA input. In this study, a multiplex panel of 27 genes was used to generate an expression profile that, when compared to human ESCs, characterizes and defines directly reprogrammed human iPSCs derived from skin fibroblasts.
A multiplex gene-expression assay, based on traditional RT-PCR, incorporates a patented universal priming strategy to amplify, detect, and quantify up to 30 gene targets in one reaction. This new method, called eXpress Profiling PCR (XP-PCR), is one of several applications available with the GenomeLab™ GeXP Genetic Analysis system offered by Beckman Coulter.
Multiple sets of chimeric primers, consisting of gene-specific primer linked to the 3´ end of a universal tag sequence (Figure 1), are utilized in reverse transcription and the first few rounds of PCR amplification. The gene-specific sequence of each set of primers is designed such that an amplicon of unique size is generated for a given gene target.
Once universal tag sequences have been incorporated into the amplicons, the fluorescent dye-labeled forward and unlabeled reverse universal primers (present at a 60:1 ratio relative to the chimeric primers) dominate the priming for these targets in the remaining cycles of amplification. This results in the relative and equivalent amplification of all the gene targets in the reaction, despite differences in size and initial gene-specific priming sequence.
The fluorescently labeled PCR products are separated based on size through capillary electrophoresis and each uniquely sized fragment is identified. The fluorescent signal is quantified and then converted to a gene-expression value that is normalized to one or more housekeeping genes, included in the multiplex. The final normalized gene-expression value is used to compare levels of gene expression between samples.
The goal of this study was to establish a multiplex gene-expression assay that would characterize the pluripotent marker expression in iPSCs derived from human fibroblasts. Previous studies demonstrated that the ectopic expression of the four Yamanaka factors (OCT4, cMYC, KLF4, and SOX2), or the four Thomson factors (OCT4, SOX2, LIN28, and NANOG), is sufficient to induce pluripotency in human somatic cells.
The creation of a human iPSC line (4YA) by transducing BJ fibroblasts with a combination of retroviral vectors (pMXs) containing the four Yamanaka factors was previously reported. Additionally, BJ fibroblasts were freshly infected with these four reprogramming factors plus two others—pMXs-LIN28 and pMXs-NANOG.
RNA was extracted from the newly transduced BJ fibroblasts, the 4YA iPSC line, separately cultured CA-1 human embryonic stem cells, and mouse embryonic fibroblasts. The RNA extracts were treated with DNase and quantified.
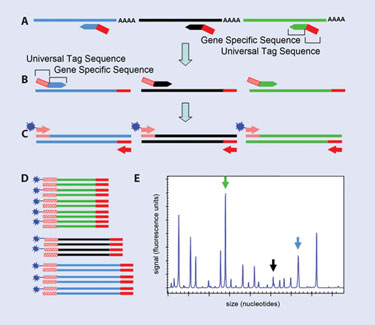
Figure 1. XP-PCR
Twenty-seven genes of interest (Figure 2) were analyzed in these samples, including the six virally encoded reprogramming factors, their six endogenous counterparts, ten pluripotency markers (MYBL2, DPPA4, ZFP42, PHC1, SALL4, DPPA2, DNMT3B, GDF3, STELLA, and TERT), and five housekeeping genes (TBP, PHC1, CCNG1, GUSB, and GAPDH) for sample normalization. A primer set for the internal control Kanamycin-resistance gene (KanR) was also included in the design and analysis.
The XP-PCR assay was performed with 25 ng of sample RNA per reaction using the GenomeLab GeXP Start Kit per the manufacturer’s protocol. The same RNA samples—excluding the reverse transcription step—were used as a negative control. The PCR products were separated and analyzed by capillary electrophoresis using the GeXP system.
We confirmed that no peaks were detected in RT-minus samples and only a KanR peak was detectable in mouse embryonic fibroblast control as expected (data not shown). Raw fluorescent signal from each experimental and control sample was normalized to the internal control gene (KanR), and the gene-expression value was calculated using a standard curve generated with GeXP Quant Tool software.
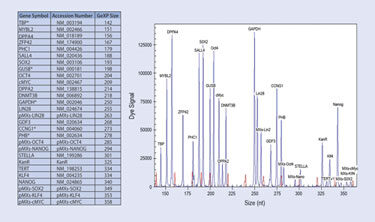
Figure 2. iPSC multiplex—gene list and expression profile
Three of the five housekeeping genes (CCNG1, GAPDH, and TBP) were determined to be the most stably expressed between the samples and were used as sample reference genes to generate normalized gene-expression values for the 27 genes in each of the three samples (Figure 3).
By comparing the expression patterns of the three sample types, it is clear that the iPSCs have a gene-expression pattern that closely resembles that of the hESCs, and yet is strikingly different from that of the parental fibroblasts (Figure 3). Specifically, the endogenous pluripotency genes are similarly active in the hESCs and iPSCs, but the expression of these genes is absent or negligible in fibroblasts. Further, it is evident that retroviral expression of reprogramming factors (e.g., pMX-SOX2) is strong in the freshly transduced fibroblasts, but silenced in the iPSCs, an expected feature of bona fide iPSCs.
By assaying the expression of 27 genes in one reaction, one is able to quickly and efficiently identify which cell lines activated pluripotency marker genes and silenced retroviral transgenes, aiding in the selection of candidate lines for further characterization. This analysis is advantageous compared with performing multiple gene-expression analyses, in terms of time and expense.
Custom iPSC multiplexes designed for use with the GeXP system are powerful tools for monitoring the activation of pluripotent markers and the reprogramming status of iPSCs. Similar types of gene panels can be used to efficiently monitor the quality of iPSC or ESC cultures during maintenance and expansion, as well as subsequent differentiation into specific cell lineages. Additionally, this method can be effectively applied to similar studies that involve gene-expression analysis and biomarker detection.
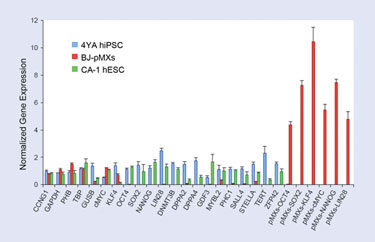
Figure 3. Gene-expression results from the iPSC multiplex
Kathryn Sciabica, Ph.D. ([email protected]), is senior applications scientist at Beckman Coulter. Web: www.beckman.com. Knut Woltjen, Ph.D. ([email protected]), is manager, and Akitsu Hotta, Ph.D. ([email protected]), is postdoctoral fellow at Ontario Human iPSC Facility. The GenomeLab GeXP Genetic Analysis system is for laboratory use only, not for use in diagnostic procedures.



