April 15, 2011 (Vol. 31, No. 8)
Researchers Take Aim at the Four Separate Hurdles that Stand Between Success and Failure
Despite the Nobel Prize-winning discovery of RNA interference (RNAi) over a decade ago and the billions of dollars spent developing therapeutic applications, delivery issues continue to challenge the field. To address these problems, researchers are creating novel strategies to achieve a safe and effective mode of delivery.
New approaches were highlighted at the “Oxford RNAi Conference” held last month in the U.K. and will also be presented at Select Biosciences’ “RNAi and miRNA World Congress” to be held later this month.
Emerging strategies include the use of nanoparticles and nanotubes as carriers for the short interfering RNAs (siRNAs) that deal the blow of gene knockdown via mRNA degradation. Scientists also are attempting to refine homing strategies for delivery utilizing the exquisite specificity of antibodies.
Xavier de Mollerat du Jeu, Ph.D., staff scientist at Life Technologies says there are three problems that need to be solved before siRNA advances to the clinic, “delivery, delivery, delivery”, referencing the well-known quote once made by gene-therapy pioneer Inder Verma, Ph.D. This has been and continues to be the key challenge.
The problem is that there are four barriers that a successful siRNA therapeutic must overcome. Once introduced into an organism, patrolling macrophages and cells of the reticuloendothelial system see the siRNA complex as foreign and attempt to degrade and eliminate it. A second important hurdle is the need to target the siRNA to the proper organ/cell of interest. Thirdly, once inside the cell the siRNA must be able to escape the endosome pathway that also attempts to degrade it. Finally, once the siRNA overcomes those obstacles, then it must be potent enough to accomplish knockdown.”
So far the most promising results in vivo were obtained with liposomes, according to Dr. de Mollerat du Jeu. “The field has achieved a great deal of success in utilizing siRNA in cultured cells. Translation to in vivo delivery has been more of a challenge. Our company has developed lipid-based nanoparticles for in vivo animal work. We recently launched Invivofectamine® 2.0 Reagent. After a single intravenous injection of 5 mg/kg FactorVII siRNAs complexed with Invivofectamine® 2.0, we observed 90 percent mRNA and protein level reduction in the liver for more than three weeks.”
There are other potential time and money-saving applications for this technology. “There are many uses, especially as a substitute for transgenic mice for target validation and ADME studies. Currently, researchers must develop expensive animal models that knockout a certain gene in order to determine its mechanism of action. However, the use of Invivofectamine with an appropriate siRNA could achieve the same results and also demonstrate the mechanism of action in much less time and at a fraction of the cost.”
Invivofectamine can multitask, says Dr. de Mollerat du Jeu. “Another useful feature of Invivofectamine is the ability to knock down up to four targets at once. This is a very powerful way to dissect whole pathways for assessing many interactions at once. For the future, we continue to create new applications for our reagent such as for liver cancer, and envision new uses by changing the route of delivery such as direct injection into the brain or tumors.”
Carbon Nanotube Delivery
Another siRNA nano-based delivery strategy is the use of long cylindrical fibers called carbon nanotubes. Interest in carbon nanotubes developed after the discovery that carbon could form stable and ordered structures other than diamonds and graphite. Thousands of papers have been published on these remarkable and versatile structures that range in diameter from the nanometer to micron range.
A key advantage of carbon nanotubes is that they can act like nanoneedles, easily piercing the cell membrane to deliver the goods, says Kostas Kostarelos, Ph.D., professor in the Nanomedicine Laboratory, Centre for Drug Delivery Research, The School of Pharmacy, University of London.
“There are fundamental differences between nanoparticles and carbon nanotubes. While spherical nanoparticles have been used in the last few decades, we are now finding that fiber-shaped nanotubes have dramatically different interactions with biological matter, such as the plasma membrane. In cell culture, carbon nanotubes can deliver siRNA directly into the cytoplasm with orders of magnitude higher levels than nanoparticles.”
“The challenge for biomedical applications has been the insolubility of carbon nanotubes in most buffers in general, and in biological fluids in particular. To overcome this they have been coated with amphiphilic molecules (lipids and polymers) or functionalized with various chemical groups that can vastly improve their water dispersibility. But the degree of aggregation and individualization of nanotube materials in biological fluids such as blood and interstitial fluids have important roles in their pharmacological performance.”
Other challenges also need addressing. “One caveat is that the gene knockdown we see is not commensurate with the amount of siRNA delivered inside the cell,” Dr. Kostarelos reports. “Our working hypothesis is that the siRNA is not being efficiently released from the nanotube. We and others are working to prepare constructs with improved detachment capabilities.”
Nanotube vectors are utilized by the Nanomedicine Lab for the local delivery of siRNAs in the central nervous system. “The beauty of this approach is that we can administer the therapy directly at the loci in the brain where it is needed. Think of it like the extension of a syringe at the nanoscale. In animal models we were able to rescue stroke-damaged brain tissue in mice using this approach. Other applications we are working on include Parkinson disease and brain cancers.
“We are still in the early stages of this technology, but hope our work will act as an impetus to further explore and, ultimately, clinically translate the therapeutic capacity of chemically functionalized carbon nanotubes.”
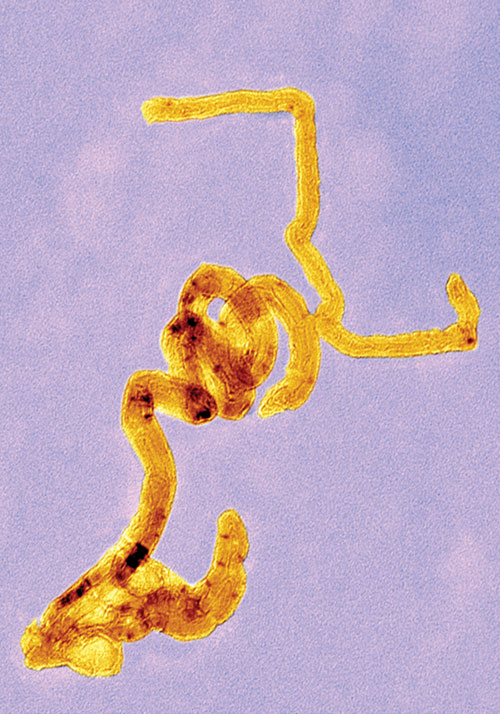
A key advantage of carbon nanotubes that researchers at the University of London are exploiting is that they can act like nanoneedles, easily piercing the cell membrane to deliver siRNA.
Targeting Lung Cancer
EGEN is focusing on synthesizing biocompatible delivery vehicles to deliver genes as well as siRNAs. “We are utilizing a variety of different delivery approaches in our TheraSilence™ platform that are designed specifically for in vivo RNAi applications including lipids, polymers and lipopolyamines,” according to Jason Fewell, Ph.D., vp of preclinical R&D. “In our lipopolyamine nanoparticle systems we use a single lipid-based core structure with a flexible head group that allows for modification by covalent attachment of functional groups such as polyethylene glycol and targeting ligands. We can then dial in various ratios of the core structure with the modified structures to optimize delivery for different applications.
“This approach provides for controlled formulation conditions and is distinct from other technologies that use multiple different lipid types in a single formulation. The advantages we find with our system are that it creates a more uniform structure, simplifies the manufacturing process, and provides consistent advantages in uptake.”
The company describes new results that employed a murine model of lung cancer. “Our preliminary studies indicated preferential uptake and retention of siRNA in the lung endothelium following intravenous administration of our lipopolyamine system. As proof of principal we subsequently targeted STAT3, which is involved in the cellular processes of apoptosis and proliferation and is implicated in a variety of cancers, especially the lung. We found that the administration of STAT3 siRNA provided sustained knockdown of STAT3 transcript and resulted in decreased tumor growth. When delivered intravenously, the toxicity was low and well tolerated. We monitored liver transaminases, liver toxicity, lung histopathology, and various cytokine markers of inflammation.”
Dr. Fewell reports they will next pursue increasing dosing regimens to get a more robust knockdown. They also want to optimize treatment regimens as well as evaluate this delivery system as a potential therapy for additional diseases of the lung with the goal of rapidly moving the technology into preclinical development.
“We have already learned much about nucleic acid based drug development utilizing our TheraPlas™ plasmid DNA delivery platform. The latter provides for the local delivery of IL-12 gene for treatment of tumors in the peritoneal cavity.” The company’s lead product, EGEN-001, is currently in Phase II trials for ovarian cancer.
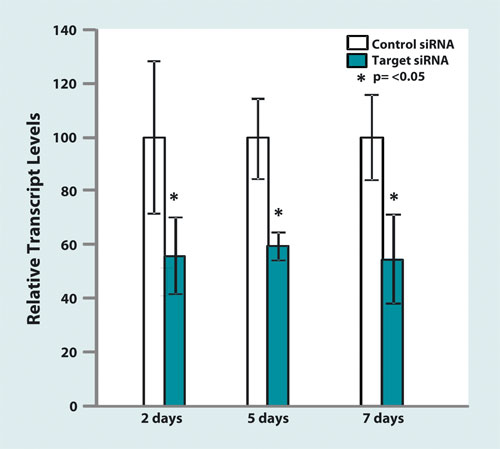
siRNA-mediated transcript knockdown in lungs: Persistent target gene knock-down in mouse lung following a single IV injection of siRNA (2.0 mg/kg) formulated with EGEN’s TheraSilence™ lipopolyamine. RNA was isolated from whole lung homogenates and analyzed by qRT-PCR. Transcript levels of animal injected with the active siRNA are compared to animals that were injected with a nonsilencing siRNA control seqence.
Targeting Leukocytes
Leukocytes are implicated in inflammatory diseases, blood cancers, and leukocyte-tropic viral infections. However, systemic delivery to leukocytes is complicated by their resistance to conventional transfection methods and to their dispersal throughout the body. Dan Peer, Ph.D., head, laboratory of nanomedicine, Tel Aviv University, and colleagues created a novel approach that exclusively targets integrins of leukocytes using nanoparticles armed with specific monoclonal antibodies and entrap siRNAs.
“Often cationic-based nanoparticles have been utilized for delivery of siRNAs. But these can also produce a set of adverse effects such as cytokine storm that causes serious flu-like symptoms. It’s not just about delivery, it’s also about safety. We, instead, developed neutral lipid-based nanoparticles (~80 nanometers) surface functionalized with a polysugar called hyaluronan, a naturally occurring glycosaminoglycan. These biopolymers are safer and stealth-like to the immune system. They also create a scaffold for the attachment of antibodies or antibody fragments. Because this is a platform technology, one can change the targeting antigen, much like a GPS system, by changing the homing antibody.”
Dr. Peer cites the example of inflammatory bowel disease (IBD). In this case, particles are loaded with a monoclonal antibody against a gut-specific integrin called β7 integrin. “Using our I-tsNP (integrin-targeted stabilized nanoparticles) we identified cyclin D1 (CD1) as a potential novel target for IBD. CD1, a cell-cycle regulator, becomes upregulated during IBD in both epithelial and inflammatory cells. We utilized a mouse model and found that leukocyte-directed CD1 siRNA inhibits the intestinal inflammatory response.”
According to Dr. Peer, other applications include viral diseases that affect lymphocytes such as HIV. “In this case I-tsNP surface modified with LFA-1 integrin monoclonal antibodies were employed to deliver CCR5-siRNAs to human lymphocytes and monocytes. This system was shown to protect mice from HIV infection and did not induce an interferon response or secretion of TNF-alpha (an inflammatory cytokine).”
For the future, Dr. Peer plans to study gene-expression patterns to help identify novel targets. “We want to better understand the mechanisms and gene-expression patterns between patient and normal samples. We are already learning a lot from such studies.”