July 1, 2016 (Vol. 36, No. 13)
Three-Dimensional Cell Culture Has Two Dimensions, the Inward and the Outward
The era of three-dimensional (3D) printing has arrived—not just the 3D printing of uniform solids, but the 3D printing of heterogeneous, living tissues. If you are content with producing inert objects, you can use the 3D printers sold at Barnes & Noble.
But if you want to construct human tissue, you will need a bioprinter, a device that can lay down living cells in patterns that are correct in exacting detail. Fortunately, bioprinters are becoming commonplace, and the applications that they enable—such as 3D cell cultures—are starting to go mainstream.
The global market for 3D cell cultures is poised for explosive growth. According to BCC Research, it will reach $2.2 billion by 2019. This growth, which is supported by an unprecedented pace of innovation, is a response to the increased demand for safety testing in drug discovery.
Advances in 3D cellular design were showcased at the recent 3D Models and Drug Screening Conference, which was organized by the Global Technology Community. The event, which was held in Berlin, highlighted the use of bioprinters to construct human liver tissue; the genetic manipulation of stem cells to create mini-brains, and the development of tools and imaging technologies for high-content phenotypic screening and validation. Applications discussed at the meeting ranged from drug design to safety testing, and from cancer therapeutics to regenerative medicine.
Bioprinting 3D Liver Tissue
For many years, scientists have struggled to capture the form and replicate the function of native tissues. Although native tissues possess subtleties that still elude 3D culture systems, scientists are making progress. For example, scientists at Organovo have utilized bioprinting technology to create a new 3D model of human liver tissue and move beyond the limitations of animal models and 2D cell culture.
“Although animal models help determine the acute, high-dose toxicity of a drug being evaluated, the problem is the unpredicted toxicity that is not detected in animals,” commented Sharon Presnell, Ph.D., Oranovo’s CSO. “Another problem is that conventional 2D cell culture models do not accurately reflect the complex microenvironment of liver tissue.”
The company’s exVive3D™ human liver tissue model is constructed from three basic cellular elements: primary hepatocytes, primary endothelial cells, and primary stellate cells. “Once a tissue design is established and a protocol is put in place, the multicellular ‘bio-ink’ building blocks are then dispensed from a bioprinter, using a layer-by-layer approach that is scaled for the target output,” Dr. Presnell said. “The automated bioprinting process allows spatial control such that tissue-specific patterns or compartments can be quickly produced that mimic key aspects of in vivo native tissues.”
It takes about 30 minutes to bioprint the beginnings of tiny livers into a 24-well plate. These mature into liver-like tissue in about 60 hours and can be maintained for at least six weeks. “They maintain a tissue-like density,” asserted Dr. Presnell. “They have highly organized cellular features, such as intercellular tight junctions and microvascular networks.”
To examine drug-induced liver injury, Organovo employed its model while using valproic acid (VPA), a compound known to induce liver injury in humans. The company found that tissues treated with VPA daily for 14 days showed clear evidence of dose-dependent oxidative stress and tissue death as assessed by glutathione and adenosine triphosphate assays, respectively, and clinically relevant histological changes including the deposition of fat. Organovo concluded that its exVive3D human liver tissues effectively modeled multiple modes of VPA-induced liver injury.
Organovo reports that it is working on additional models. “We expect,” Dr. Presnell revealed, “that our kidney proximal tubule model will be available the third quarter of 2016.”

A tissue engineer at Organovo loads bioink into one of the company’s bioprinters. The procedure is part of a process to generate functional 3D human tissue models for preclinical testing and drug discovery research. One such model is Organovo’s exVive3D Human Liver Tissue.
Constructing Human Mini-Brains
With the increasing incidence of neurodegenerative disorders such as Alzheimer’s disease, multiple sclerosis, and Parkinson’s disease, researchers are testing 3D cellular models that could be superior to animal studies because the cells are derived from humans instead of rodents. Researchers at Johns Hopkins Bloomberg School of Public Health created “mini-brain” organoids composed of neurons and glia cells that closely mimic the brain’s cellular composition.
“We used induced pluripotent stem cells (iPSCs) derived from the skin of healthy adults,” explained Lena Smirnova, Ph.D. “Then we genetically programmed them to an embryonic stem cell–like state.
“These cells are then grown over a period of eight weeks into very small mini-brains of about 350 microns in diameter consisting of different types of neurons as well as astrocytes and oligodendrocytes (to serve as supports). Oligodendrocytes produce myelin that helps insulate the axons of the neurons to allow for rapid communication.”
As evidence that the cells were recapitulating real brain tissue, the Johns Hopkins team monitored electrophysiological activity with electrodes. The team, which used a setup similar to that used by an electroencephalogram, found spontaneous electrical activity.
“Obviously, the system could be useful in drug development,” said Dr. Smirnova. In support of this contention, she referenced the system’s reproducibility. “Hundreds to thousands of exact copies of the organoids can be derived in each batch providing a highly standardized model. However, there are many other applications including studying central nervous system mechanisms, neurotoxicity reactions, and brain development.”
Further, mini-brains can be developed from patients as well as from healthy individuals. “We can generate a mini-brain organoid bank by reprogramming fibroblasts from the skin of patients with different neurological diseases,” asserted Dr. Smirnova.
The leader of the Johns Hopkins team, Thomas Hartung, M.D., Ph.D., is applying for a patent. Besides serving as a professor of evidence-based toxicology at the Bloomberg school, Dr. Hartung is a cofounder of Organome, which aims to produce mini-brains for distribution.
Optimizing the 3D Environment
Investigators setting up a 3D cell culture system have many decisions to make as to which culture conditions provide the most accurate biology for their particular cell type. “One of the frequent questions investigators ask is what matrix and coating concentration is optimal for their cell type,” noted Marshall Kosovsky, Ph.D., global scientific support manager, Corning Life Sciences. “The first advice I can give them is to do a thorough literature search regarding the biology of their cell type.”
“They need to understand the key requirements for cell behavior—including interactions at the level of extracellular matrix (ECM) molecules and growth factors—and the nature of previously used systems,” continued Dr. Kosovsky. “Next, it is important to clearly define their experimental objectives. For example, should they culture one cell type or mix multiple cell types to more closely reflect the situation in vivo? Finally, it’s important to fully research the spectrum of materials and functionality of reagents available for 3D culture.”
Corning Life Sciences, one of the leaders in the field, provides a wide portfolio of products and technical/manufacturing expertise for 3D cell culture. “Natural ECM-based hydrogels (such as Matrigel matrix and Collagens) and systems for generating 3D cell aggregates known as spheroids (such as the Corning Spheroid Microplate) are widely employed for 3D cell culture,” asserted Dr. Kosovsky. “Both model systems provide environments that support a variety of applications including tumor cell biology, stem cell differentiation, and drug discovery.”
Systems that incorporate permeable supports provide another approach for 3D cell culture. In such a system, a tissue culture well consists of two chambers separated by a microporous permeable membrane.
“The use of such cell culture inserts provides a very dynamic platform for 3D cell culture,” explained Dr. Kosovsky. “Cells can be seeded on three different surfaces: the top of the membrane; the bottom of the membrane; and the floor of the well. Further, researchers can choose to coat one or more of these surfaces with a variety of 3D matrices.
“These options give researchers a great deal of flexibility,” added Dr. Kosovsky. “Researchers can mix and match surfaces and cell types to optimize the 3D environment for the specific application.”
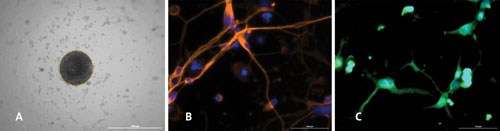
Human neural stem cell–derived neurospheres generated in the 96-well Corning Spheroid Microplate (A) exhibit multipotency and differentiate into neurons (B), oligodendrocytes (C), and astrocytes (not shown).
Culturing with 3D Microcavity Arrays
One of the problems with hydrogels and other matrices, said Eric Gottwald, Ph.D., CEO, 300Microns, is that the size of the aggregates cannot be accurately controlled. He added that matrices may interfere with microscopic applications by compromising image quality due to diffraction artifacts.
To address these challenges, 300Microns developed a matrix-free technology called microcavity arrays. These are manufactured using very thin polymer films (50 microns before microthermoforming, 7–10 microns after thermoforming) in a highly parallel fashion with a technique called microthermoforming.
“Thermoforming has been known from the macroscopic world for more than 50 years,” noted Dr. Gottwald. “Only recently has it become possible to downscale the thermoforming process to the micrometer range.”
The downscaled process, microthermoforming, consists of multiple steps: heat a thin polymer film to a softened but still solid state; apply pressure so that the film assumes a three-dimensional form that is determined by a micromold; cool the film so that the film “deforms,” that is, takes its final shape apart from the micromold.
The microthermoforming of a film gives it microcavities, which can easily be constructed with various geometries, asserted Dr. Gottwald. These microcavities are typically about 300 microns in diameter and up to 300 microns in depth. Why 300 microns? This is the maximum distance between two adjacent capillaries in mammalian tissue, and it provides for more natural 3D oxygen diffusion.
The microcavity arrays house between 10,000 and 500,000 cells in up to 169 3D aggregates per standard well of a 96-well plate. “These offer superior advantages in high-throughput and high-content screening applications,” insisted Dr. Gottwald. “They allow detailed imaging. Also, because of the known position of each microcavity in the array, automated microscopy is much more time saving as compared to current spheroid-generating techniques.”
300Microns’ 3D models have been employed for a variety of applications such as generating IC50 curves of hepatocytes, differentiating iPSCs into beating heart muscle cells, and stem cell maintenance of hematopoietic stem cells in co-culture with mesenchymal stem cells. “Since we are not limited in size and geometry, even whole organism screens (zebrafish) could be realized,” concluded Dr. Gottwald. “The list of possible applications can be extended even further.”
Animal-Free Cosmetic Testing
The skin of organisms provides an essential permeability barrier to the external environment. Indeed, terrestrial life requires maintaining the integrity and cohesion of the outermost layer of epidermis, the stratum corneum. Without it, water would evaporate from our bodies and various antigens could penetrate easily into the skin. Defects in this system from cornification and barrier anomlies underlie a clinically diverse set of skin disorders.
Theodora Mauro, M.D., professor of dermatology, University of California, San Francisco, and Dusko Ilic, M.D., Ph.D., reader in women’s health, King’s College (London), wanted to develop a functional model for better understanding the molecular events in skin biology, mechanisms of diseases, drug and cosmetics screening, and for development and validation of novel therapies.
“We had two goals,” recalled Dr Ilic. “The first was to design highly reproducible and easily scalable models. The second was to adapt these models to current Good Manufacturing Practices and production under animal product-free conditions.
“We generated a 3D model for human epidermal equivalents (HEEs) utilizing human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs). These primary cells are capable of infinite proliferation and bear a fully characterized genetic footprint. Our 3D HEE model from hESC/hiPSC-derived keratinocytes retained all of the cellular strata and normal human skin barrier properties identical to that seen in the human epidermis. Further, it can be easily scaled up for use in regenerative medicine, drug development, and aesthetic medicine.”
According to Dr. Ilic, this latter use is important. “Development of novel animal-free testing strategies was boosted since March 2013,” he explained. “At this time, the European Union introduced a complete ban on the sale of all new cosmetics that had been tested on animals.
“Our model is ideal for screening of cosmetics ingredients. All other commercially available in vitro 3D models for cosmetic testing do not have a functional permeability barrier and, therefore, cannot predict penetration rate, toxicity, etc. All tested compounds will go right through the barrier, which is not the case in healthy skin.”
Modeling the Most Virulent Cancers
The malignancy of cancer often originates from the existence of a small subpopulation of stem cells that are particularly recalcitrant to cancer therapy. “Cancer tissue can be divided into differentiated and stem-like cells,” advised Esmaiel Jabbari, Ph.D., professor of chemical and biomedical engineering, University of South Carolina. “While the bulk of a tumor consists of differentiated cells, which are sensitive to chemotherapy, the smaller fraction consists of stem cells, which are resistant to therapy and remain in the tissue.”
Dr. Jabbari leads a research team that is developing 3D models enriched in such malignant stem cell subpopulations. “We are studying how matrices can be used to manipulate or tune the cell microenvironment (niche) with respect to the physical, mechanical, biochemical, and cellular properties,” he noted. “We are learning how to enrich the cancer stem cell population.”
Dr. Jabbari’s team encapsulates cancer cells from various tissues into the various matrices. “The niche enriches growth of stem cells and destabilizes growth of differentiated cancer cells,” he explained. “In a recent study, we developed a model using polyethylene glycol diacrylate (PEGDA) hydrogel and found a link between stiffness of the matrix and stem cell growth and marker expression.”
The most immediate application of the model is for drug screening. “Current screening protocols evaluate drug toxicity against differentiated cancer cells that are far less invasive than cancer stem cells,” stated Dr. Jabbari. “Our approach will enrich for cancer stem cells of malignant tissue and then screen drugs specifically against this most invasive stem cell population. We are looking to form partnerships with biopharmaceutical companies.”



