June 1, 2010 (Vol. 30, No. 11)
Novel Instruments, Comprehensive Services, and Streamlined Assays Boost Scientists’ Efforts
Multiplexing is a critical component of rapid screening technologies. At the annual meeting of the Society for Biomolecular Sciences held recently in Phoenix, a number of industry and academic scientists explained their pursuit of novel technologies that allow assays to be performed simultaneously on each well of a microtiter plate. Label-free technologies such as surface plasmon resonance (SPR) are available through various companies, and several contract research organizations are adept at their application.
Helena Nilshans, senior market director for life sciences at GE Healthcare, talked about the Biacore™ 4000 LMW extension package, which she reported “is mainly designed for small molecule drug discovery applications. Backed up by dedicated software tools for screening of fragments and other low molecular weight compounds, Biacore 4000 supports a range of assays from screening to characterization applications such as lead optimization.”
According to Olof Karlsson, Ph.D., a senior scientist in the Biacore R&D division, the amount of data generated on a conventional SPR system takes days to sort out with ordinary software. But with the Biacore 4000, less than two hours are required. “Not only the speed of the processing, but the overall quality of the data analysis is notably improved.”
Dr. Karlsson shared data detailing the multiplexing features of the Biacore 4000; he said that 16 targets can be run in parallel, processing up to 4,800 interactions in 24 hours. Both hardware and software are optimized for efficient large-scale assays, permitting 60 hours of unattended run time, he added. The system also includes an antibody-analysis package, allowing several interactions to be studied simultaneously.
Low molecular weight pharmaceuticals are far from an endangered species. “The vast majority of drugs are still small molecules, whose cost and convenience guarantee that they won’t soon be replaced by biologics. Biacore technology allows us to use information concerning specific structures, expanding the description from a small fragment into a model of a larger molecule, optimized for binding to its target.
“The software provides parameter analyses and identifies deviations. Elimination criteria start with the new data from a run and eliminate the bad data first, allowing us to promptly focus on the good candidates.”
Focusing on Targets
BioFocus offers a range of discovery services, principally aimed at target validation and developing molecules against targets, according to Doris Hafenbradl, Ph.D., senior director of biology and natural products. The company just reported the availability of four biologically focused libraries containing drug-like compounds that are aimed at kinases and protein-protein interactions. These compounds are modeled to bind to the kinase hinge, with allosteric sites, or to enter into other unspecified binding modes on the molecule.
“We use surface plasmon resonance extensively in our screening platforms,” Dr. Hafenbradl explained. “This is especially relevant for screening of our diverse fragment library in which label-free technologies are especially convenient.
“We are also well-positioned for designing drugs using our chemo-informatics library of 900,000 compounds. Using high-throughput screening we have identified a large range of novel targets.” The use of radioactive tags is no longer a popular strategy in molecular analysis, but it has to be the starting point. “If you have an enzyme that is suspect as a candidate for a therapeutic role, you can find compounds that will bind to and inhibit it.”
BioFocus also maintains an active screening program for epigenetic targets. Epigenetic modifications of the DNA form the basis of much phenotypic expression, and a wide range of disease states are now known to be associated with a group of modifications to DNA and the histone chromosomal proteins that include histone phosphorylation, methylation, acetylation, ribosyl transfer, ubiquitinylation, and proteolytic-driven cleavage.
In this case, BioFocus uses an “intelligent selection” of library subsets for primary screening, rather than employ a large compound library, which would be more appropriate for an unknown target. The narrowing process uses in silico modeling of the enzymatic site in accordance with ADME. These parameters allow the 3-D structural information concerning the target to be maximized.
“We are gradually accumulating a body of experience that will allow the elimination of futile compounds,” noted Dr. Hafenbradl.
A case study on histone kinase detailed the use of a targeted library of 60,000 members, which resulted in 82 validated hits after several rounds of verification. According to Dr. Hafenbradl, this project established the validity of the firm’s fast-track approach to novel chemical entities capable of inhibiting epigenetic targets.
In addition to their ability to uncover drug targets, BioFocus screening platforms have the potential to uncover disease biomarkers that could be the subject of multiplex analysis, using label-free detection such as SPR, Dr. Hafenbradl concluded.
Cellular Responses
Genetix’ CellReporter™ system was developed for assessing cellular responses, according to Jerry Williamson, president of U.S. operations. This technology can be used to study a variety of cell functions such as cell-cycle analysis, effects of cytotoxic compounds on cell function, and the process of protein translocation.
Through the use of flexible image-analysis software, each object can be characterized, and individual cell responses can be identified. A number of assays can be run simultaneously for multiplexing functions.
Tobin Dickerson, Ph.D., of Scripps Research Institute, is using a reconfigured CellReporter instrument to isolate compounds that block the toxic effects of botulinum neurotoxin. “The cell-based assay is an excellent format to analyze inhibitors of botulinum neurotoxin.”
This extremely potent toxin is fatal to humans at a dose of 1 ng/kg. Its mechanism of action is through inhibition of exocytosis in neurons. Rather than outright killing, the toxin acts by internalizing and brings transmission within the nervous system to a grinding halt. The search for an effective small molecule inhibitor has been unsuccessful since most drug screens search out compounds that prevent cell death.
As part of his approach, Dr. Dickerson screened a library of small molecules for allosteric modulators, as well as direct inhibitors. The screening system he uses reportedly works in a precise fashion, and his team has isolated a number of small inhibitory molecules. Two of these compounds are now being evaluated in animal models.
Detection System
Jas Sanghera, Ph.D., commercial director of TTP LabTech, introduced the company’s new technology. “Mirrorball, which is based on an array of mirrors, will complement our existing product line.”
The Mirrorball configuration was developed as a result of input from the antibody discovery industry. The instrument is based on flow cytometry because of its ability to detect low-abundance antigens, including cell surface proteins. According to Dr. Sanghera, until now laser-scanning cytometers have not been able to provide the necessary sensitivity required for mix-and-read assays.
“Mirrorball’s microplate cytometric technology make this an effective system for high-throughput antibody screening. While simultaneous laser scanning ensures that Mirrorball has the requisite multiplexing and analytical capabilities, the laser-scatter channel provides an independent method for cell- and bead-based identification. This design permits improved sensitivity when multiplexed with fluorescent reporters.
“As the pharma industry is moving to biologicals as new drug entities, a rapid data-analysis platform is increasingly important. The Mirrorball scans the entire well, producing a true representation that allows you to see how the cells are being distributed. No other instrument can do that.”

Mirrorball is a new laser scanning microplate cytometer that TTP LabTech says it designed with the needs of the antibody discovery industry in mind. It is reportedly sensitive enough to be able to detect low abundance antigens, works with the mix-and-read assay format, and can perform assays in a microplate format.
Receptor Investigation
Rapid and sensitive assays for cellular receptors are in demand for both clinical and basic science applications. Cisbio has developed a line of products based on FRET.
Tag-lite assays use a relatively undifferentiated cell line that provides a wide-ranging foundation for measuring cellular function, according to the company. The SNAP-tag labeling procedure (New England Biolabs) is used to couple a cryptate fluorophore to the receptors on the cell surfaces. Addition of a ligand carrying the second fluorophore will result in a powerful signal, forming the basis of this homogeneous assay, which requires no washing, much like ELISA-based procedures.
Tag-lite has been engineered for highly selective ligand binding, receptor dimerization, and functional assays. Among recently developed products are Cellul’erk, for measuring phosphorylated-ERK1/2, and the IP One Tb assay, for detecting inositol(1)phosphate, a major product of the phosphatidyl inositol cascade.
“For a given receptor, the same Tag-lite cell line can be used as a starting basis for these tests, which have all been streamlined and validated with prelabeled frozen cells,” stated Francois Degorce, director of marketing and communication.
“Therein lies the breakthrough of the Tag-lite concept—enabling receptor investigation to address multiple angles while eliminating the need to develop different cells for each.”
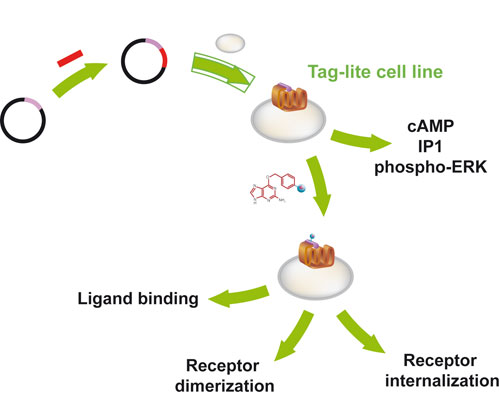
Multimode Tag-lite cell lines express SNAP-tag cell surface receptors, which remain fully functional and can be assessed through second messenger or phospho-ERK detection. When further labeled with Lumi4-Terbium, the same cells can be used for ligand binding, receptor dimerization, and internalization. [Cisbio]
Biomolecular Interactions
John Kulman, Ph.D., a principal investigator at the Puget Sound Blood Center (PSBC), described his investigations aimed at further enhancing the performance of Bio-Rad Laboratories’ ProteOn™ XPR-36 System. The device is designed for drug discovery, with a workflow that allows it to simultaneously monitor 36 interactions in real time.
“SPR is largely underutilized,” Dr. Kulman argued, “due to cost and time limitations for producing high-quality ligands, difficulties in regenerating the ligand-coupled surface between runs, and, finally, throughput limitations inherent in the instrumentation.”
Dr. Kulman said that if these limiting factors were removed from the experimental design, quantitative evaluation of the mechanism of action of a drug candidate could be much more easily integrated into a drug-development program.
“Our approach is a blend of basic science and industrial engineering design, the end result of which is much faster and cheaper throughput without compromising data quality.”
In order to simplify the workflow, Dr. Kulman developed a calcium-dependent immunocapture platform. The approach employs ligands generated by small-scale transient transfection of mammalian cells that are purified on-chip, so there is no upstream purification of ligand. Rather, the process of plasmid generation is the only rate-limiting step in ligand production. Since regeneration back to the capture agent is achieved cleanly and rapidly at millimolar concentrations of EDTA, the same capture agent can be used continuously for multiple experimental runs.
At this time, the ProteOn is not certified for clinical diagnostic applications, but Dr. Kulman believes that the throughput capacity of the instrument makes it ideal for clinical labs that process multiple patient samples. Toward this end, he and his colleagues at the PSBC are developing new diagnostic applications for a spectrum of bleeding disorders collectively known as autoimmune thrombocytopenias.
“By integrating multiplexed SPR with other high-throughput platforms such as multimode plate readers and flow cytometry, we can do some exciting stuff, we can cover a lot of ground, from biophysical studies to drug discovery and clinical diagnostics.”

Scientists at the Puget Sound Blood Center developed an on-chip purification method to quickly regenerate back to the capture agents on the chip surface of Bio-Rad Laboratories’ ProteOn platform for surface plasmon resonance (SPR) analysis.
K. John Morrow Jr., Ph.D. ([email protected]), is president of Newport Biotech and a contributing editor for GEN.



