August 1, 2009 (Vol. 29, No. 14)
ESETEC Seeks to Overcome Limitations of Periplasmic and Inclusion Body Production
The bacterial host Escherichia coli provides an attractive industrial protein production approach in terms of time and cost efficiency. Microbial production technology is straight forward, safe, and well understood. Knowledge of the genetic properties of E. coli is more comprehensive than that of any other host organism. Both the development of E. coli cell lines and the production of protein by the bacterium proceed quickly, often allowing high quantities of protein, and are therefore less cost intensive than protein production by mammalian cells. Furthermore, in contrast to mammalian cell culture, the risk of transmitting viral infections or transmissible spongiform encephalopathies is precluded.
Despite all these advantages, microbial expression systems have their limitations. Post-translational modifications such as phosphorylation and glycosylation are difficult to achieve in a bacterial host but are required for a number of proteins, e.g., full-length antibodies. Also, proteins with large and complex structures (e.g., heterodimers and proteins with disulfide bonds) are often challenging.
As a result, the production of complex, recombinant proteins in E. coli may produce inactive, aggregated proteins (inclusion bodies) that accumulate in cells. Although expression yields and the separation of inclusion bodies from cell debris are efficient, converting inclusion bodies into native, biologically functional proteins requires their solubilization with chaotropic agents and a subsequent refolding step.
Refolding, however, is frequently the rate- and yield-limiting step for protein production and purification. In addition to inclusion bodies, more and more therapeutic proteins are solubly produced in the periplasm of E. coli. In these cases, the native, soluble proteins fold correctly in the oxidizing environment of the periplasm and thus, do not require refolding steps. Protein yields, however, are compromised by the limited space of the periplasm. Moreover, protein purification is strenuous because the whole cell lysate containing the recombinant protein also contains many host cell contaminants.
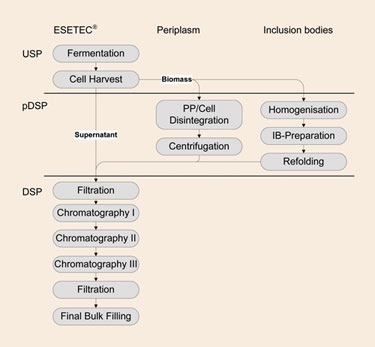
Wacker has developed an E. coli expression system—ESETEC®—that overcomes the limitations of periplasmic and inclusion-body production processes by efficiently secreting recombinant therapeutic proteins into the culture broth. By secreting the recombinant therapeutic protein extracellularly, the protein is produced in its native form. Furthermore, production yields are not limited by periplasmic volume. Refolding is not required either because the protein is expressed solubly and, since the protein has been secreted, cell disruption is unnecessary. Therefore, secretion reduces the number of necessary downstream processing steps, increases the overall process yield, and thus, lowers process costs (Figure 1).
ESETEC consists of bacterial strains that are derived from the well-characterized E. coli K12 strain of expression plasmids, and of a set of additional elements that help to optimize expression, solubility, and secretion of the target protein.
ESETEC strains are suitable for cGMP production because of their documented history. They facilitate the secretion of recombinant proteins into the culture broth thanks to their modified outer membrane. Wacker’s expression plasmids usually encode the ptac promotor and proprietary signal sequences for the translocation of recombinant proteins to the periplasm.
For translocation, ESETEC uses the Sec pathway—a major pathway of active protein transport from the cytosol across the inner cytoplasmic membrane in bacteria (Figure 2). Sec translocase recognizes the short signal sequences at the N-terminus of the protein and transports the protein across the inner membrane.
Upon transfer, a signal peptidase removes this signal sequence, and the premature recombinant protein is released with its native N-terminus. Then the premature protein is folded, and disulfide bridges are formed. Formation of disulfide bridges is favored in the oxidative environment of the periplasm, in contrast to the reducing conditions of the cytosol.
Moreover, recombinant proteins are less amenable to proteolytic degradation in the periplasm than they are in the cytosol. ESETEC thus achieves high-yield production of correctly folded proteins because these proteins are secreted into the culture broth. This system has been shown to be suitable for different prokaryotic and eukaryotic proteins, for proteins with a wide range of molecular weights and isoelectric points, for fusion as well as native proteins, for proteins with an N-terminal amino acid other than methionine, and for proteins with disulfide bridges.
High yields of up to 11 g/L have been obtained for various proteins. A range of peptides, enzymes, and proteins, such as antibody fragments (Fabs, scFvs) and engineered protein scaffolds (Anticalin®), have been successfully produced. When combining ESETEC with the high-cell-density fermentation technology DENSETEC® (another Wacker technology), optimal space-time yields and high reproducibility are achieved.
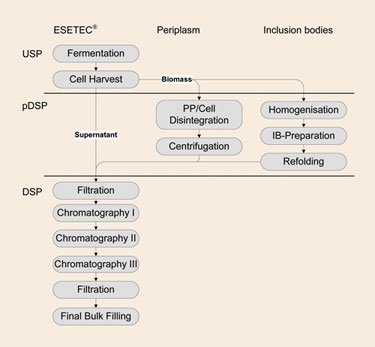
Figure 1. Reduction of downstream processing steps by ESETEC
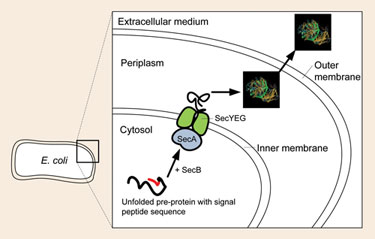
Figure 2. Transport of the unfolded premature protein containing the signal peptide for the Sec translocase (SecA) across the inner membrane into the periplasm of the cell using the Sec pathway
Antibody Fragment Production
In collaboration with MorphoSys, ESETEC proved to be highly efficient for the microbial production of antibody fragments (Fabs) from MorphoSys’ Human Combinatorial Antibody Library, HuCAL®. These Fabs have a binding affinity and therapeutic potential similar to full-length antibodies. They consist of two different polypeptide chains, the light chain and the heavy chain. Both chains have two intramolecular disulfide bridges.
By using ESETEC, both chains were correctly transferred across the inner membrane into the periplasm. They were correctly processed, folded, and assembled noncovalently into hetero-dimeric Fab molecules. These Fabs were secreted into the culture broth and remained perfectly stable during fermentation. They can be efficiently isolated and purified.
As comprehensive analysis has shown, the extracellularly secreted Fabs were fully functional and active compared to a reference Fab that had been produced by production in the periplasm. Fab yields obtained by using ESETEC were 40-fold higher than by secretion into the periplasm. Fermentation titers of more than 2 g/L were achieved without further optimization. Other Fab formats have also been successfully produced by ESETEC. Thus ESETEC is suitable for producing Fabs in high yield and quality but at lower cost than mammalian or microbial expression systems.
ESETEC produced up to 5 g/L of an engineered protein scaffold, an Anticalin owned by Pieris. Anticalins specifically bind target molecules and are an interesting alternative to antibodies because they are smaller, do not require glycosylation, penetrate tissue more efficiently, and are highly stable.
By using ESETEC, Anticalin yields have been improved 40–50 fold. Secreted Anticalin was efficiently isolated and purified from the culture broth. Two chromatographic steps were all that was needed to purify Anticalin, which was further processed by chemical modification with polyethylene glycol (PEGylation). After PEGylation, only one further chromatographic polishing step was required to achieve final product purity (Figure 3). A GMP-compliant process has now been developed.
These case studies demonstrate that ESETEC enables high product yields and simplifies downstream processing compared to other established E. coli expression systems. It also saves time and costs in the production of recombinant, therapeutic proteins.
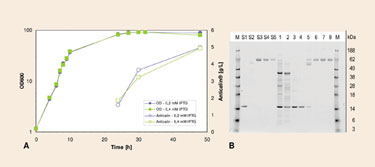
Figure 3. (A) Cell growth and product formation of an Anticalin as a function of fermentation time; (B) SDS-PAGE image of the purification of pegylated Anticalin
Merlind Muecke, Ph.D. ([email protected]), is project
manager, and Susanne Leonhartsberger, Ph.D. ([email protected]), is head of project management at Wacker Biotech.