January 1, 2011 (Vol. 31, No. 1)
Completion of the Human Genome Project Has Upped the Ante
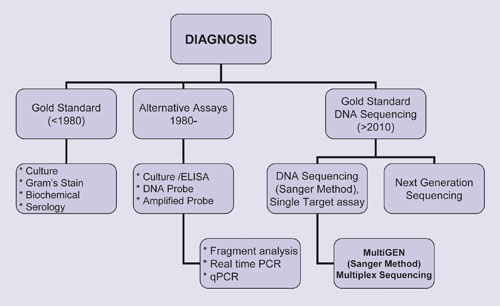
There are more than 2.7 million clinical samples processed daily in the U.S. for infectious diseases, genetic predisposition, and cancer. Currently, only around 10% of these tests are completed with commercially available test kits. The remaining samples are tested with a wide range of methods ranging from hanging drop and Gram staining to ELISA and culture. Due to their expense, molecular methods are mainly used for genetic predisposition and cancer-related assays.
This variability has created a fragmented market, where results are subject to varying degrees of sensitivity/specificity and test results are reported over a two- to seven-day period. These factors further fragment the market, increasing the cost of testing, and compromising patient care.
Completion of the Human Genome Project (HGP) led to the formation of the bioinformatics discipline, which has now become the backbone of all life science applications including diagnosis. This article reviews the new field of diainformatics, or bioinformatics for diagnostic purposes.
As a result of technological advancements, diagnosis is rapidly moving toward molecular analysis.
Nucleotides are the building blocks of all genomes, and the order in which they are linked together defines a genome and its function. It is not only the structure of DNA that must be ascertained, the order of nucleotides must be determined as well. This need was intelligently fulfilled by Sir Frederick Sanger who developed Sanger sequencing, which was instrumental in the success of the HGP.
The existence of variation within genomes is now well known. Variations include microbial targets, human genetic factors that are predisposed to diseases, and mutations that predict effectiveness to chemotherapy. Researchers now need to further their understanding of these variations in order to properly diagnose patients and manage treatment.
Historical overview of clinical diagnosis from cell-based assays to molecular diagnosis. Molecular diagnosis meets present clinical demand and provides relevant clinical information beyond identification.
Innovative Analytical Tools
Over the last four decades, a number of revolutionary analytical tools have been developed, including restriction enzyme digestion, hybridization, and PCR. Although these tools were used in the HGP, the brunt of the work was handled by Sanger sequencing. Sanger sequencing is not a panacea though; its high cost as well as the need to sequence more than one target severely limits its use in the clinical testing market.
PCR was developed primarily to produce short segments of double-stranded DNA, mainly for cloning purposes. It is able to generate millions of copies of short genomic segments, which has led to it largely replacing the traditional culture method.
Restriction fragment length polymorphisms (RFLP) rely on gel-electrophoresis analysis. Even though it is sensitive, its use of radio-isotopes has limited its potential for extensive use in clinical testing. Nucleic acid amplification techniques have been shown to have nonspecific amplification and potential inhibition by materials in clinical samples.
In order to adapt PCR to clinical settings, a number of factors needed to be resolved. When PCR is performed in a single tube, the optimum reaction condition is compromised. Further, the key enzyme, Taq polymerase, needs only two pairs of nucleotides to anneal for its 3’–5′ polymerization. This nonspecific polymerization could occur during ramping down from the denaturing step, which usually results in nonspecific amplification.
Amplification is a key part of the process as it is important to identify what is amplified. The identification step can be strengthened through the use of two labeled-probe systems. Clinical samples may, however, have more than two targets. The inability of the traditional PCR/labeled combination to fulfill this clinical need gave rise to chip technology, which is an extension of the Southern blot. Another method is focused on detection in narrow wavelengths; an example is the Luminex platform.
Real-time PCR sprung out of the “hot-air” thermocycling principle in which the dynamics of amplification are monitored graphically. The basic principle of the real-time PCR system is its hybridization of labeled probe to the target.
Platform technologies that are presently used in routine diagnostics are mainly for detection, indicating either the presence or absence of the intended target. These technologies have been validated by correlation to clinical symptoms and/or prior accepted assays.
There are, currently, only a few FDA-approved tests, and laboratory-developed tests (LDT) are mostly used for routine clinical testing. Almost all LDTs use surrogate technologies such as real-time PCR, FISH, and mass spectrometry where the results are generated in a format that cannot be verified. Hence, the culture/ELISA combination method still continues to be in demand in the clinical market.
Striving for Clinical Use of Sequencing
Because of the limitations of existing technologies, it makes sense that firms are attempting to bring sequencing platforms into routine clinical use. NGS can generate short sequences through random priming/termination. This fact makes whole-genome sequencing possible.
Next-generation sequencers are mainly used in research settings, primarily for the discovery of SNPs and their potential correlation to diseases. Capillary electrophoresis based sequencing is also being retooled with multiple amplicon sequencing by MultiGen in an attempt to make it suitable for routine testing. Generation of dia-informatics for routine testing will enable better patient management and drug development. Success of these approaches will depend upon the cost and comprehensive clinical utility.
T.V. Moorthy, Ph.D. ([email protected]), is president/CTO at MultiGen Diagnostics.



