April 1, 2012 (Vol. 32, No. 7)
With much of the low-hanging fruit in cell culture optimization already plucked, progress today is centering on how to produce more complex molecules, improving bioproduction consistency and efficiency, and developing better predictive tools for media and processing performance.
Work on multispecific antibodies isn’t new. They offer many compelling advantages, such as attacking signaling pathways whose redundancies often circumvent monospecific therapeutics. However, creating multispecific antibodies has been challenging.
Speaking at Terrapin’s “Cell Culture World Congress” last month, Ingo Gorr, Ph.D., senior research scientist, cell culture research, Roche Pharmaceuticals, reviewed an approach to developing bispecific antibodies that overcomes many past problems.
“Most anticancer drugs do not work 100 percent; there is usually some leakage,” said Dr. Gorr. “Whenever you can hit two targets in a signaling pathway your drug is likely to be more effective because you’re not allowing the cell to bypass and block the drug’s action.”
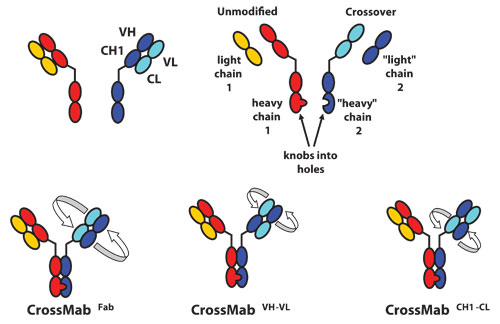
According to Dr. Gorr, Roche’s CrossMab technology can convert existing antibodies into IgG-like bispecific antibodies. Two problems must be solved to produce the desired bispecific antibody exclusively and avoid a large mixture: 1) effective induction of heterodimerization of the two heavy chains and 2) discrimination between the two light-chain/heavy-chain interactions.
To accomplish heterodimerization, Roche uses a technology invented by Genentech, which it calls “knobs into holes.”
“Our approach is to alter amino acids in the C-terminal of the antibody. Within one of the heavy chains we build a knob consisting of large amino acids, and on the respective other side we build a hole with small amino acids so that we favor or force pairing of heterogeneous heavy chains,” he explained.
Using this technique, Roche developed a bispecific IgG-like antibody for Ang-2 (angiopoietin-2) and VEGF-A. “We moved it into mouse testing where it was very efficient in preventing tumor growth,” said Dr. Gorr. Because the molecule is similar to naturally occurring IgGs, it has a similar half-life, is not prone to degradation, and is expected to exhibit reduced immunogenicity.
“We get really high titers compared to other multispecific antibody production approaches because the cell recognizes it as an antibody. We also avoid production of many side products and end up with very few product-related impurities present. Development can be done in fast timelines.”
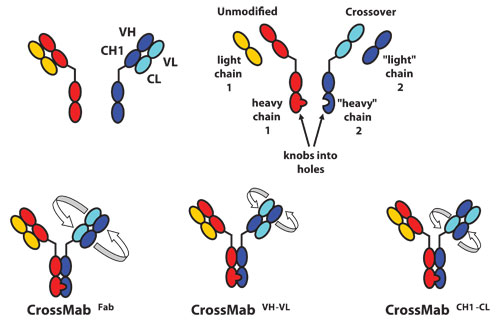
Schematic diagram of the Fab domain exchange resulting in the generation of a bispecific antibody when combined with the KiH technology. Dark colors indicate heavy-chain domains. Light colors indicate light-chain domains. (A) Both arms of the intended bispecific antibody. (B) Design of the four chains of the bispecific antibody. Heavy-chain heterodimerization is achieved by use of the KiH technology. (C) Crossover of the complete VH-CH1 and VL-CL domains. (D and E) Crossover of only the VH and VL domains (D) or the CH1 and CL domains (E) within the Fab region of one half of the bispecific antibody. [Reprinted with permission of PNAS, July 5, 2011, vol 108, no 27, 11187-11192.]
Fingerprinting Dry Media Quality
Frustration over inconsistent dry media performance occasionally roils relations between suppliers and media users.
Jörg von Hagen, Ph.D., head of process development/launch management at Merck Millipore, reviewed factors affecting dry media quality and urged cooperation between users and suppliers in adopting a fingerprinting technology—perhaps NIR—to provide rapid characterization of dry media quality.
Dr. von Hagen identified “three pillars” that determine quality: the formulation, raw material quality, and production process. To demonstrate their impact Merck Millipore analyzed powder DMEM/F12, a well-characterized media with a fixed recipe, from several suppliers.
Much of the study’s purpose, said Dr. von Hagen, was to demonstrate “it’s not just formulation that determines media performance. Particle size has to be considered as well. If you use some types of ball mill technology, you have an uncontrolled particle size reduction and end up with fairly wide range of very fine particles and some larger particles.” This in turn directly affects powder mixing and solubility—both of which affect media performance.
Several key physical-chemical attributes were reviewed:
- Appearance
- pH
- Osmolality
- Humidity
- Particle size
- Cellular performance
Wide variability was revealed in most parameters. “I wasn’t surprised,” said Dr. von Hagen. “Everybody is sourcing from different vendors or producing raw materials themselves, so you would expect differences.” More telling was that cell performance (viable cell densities) varied by as much as 50% from the fixed recipe.
Seeking a simpler fingerprinting technology, Merck used NIR to characterize the samples—sidestepping many individual tests that were often carried out. NIR results were combined with cell performance using principal component analysis. In essence, NIR spectra “summarized” many of the various individual chemical-physical components and turned out to be a robust predictor of media performance.
“You could not say by default a particular PCA score is a really good sign for all media. You need experience from historical batches,” said Dr. von Hagen. Given history, it’s possible to map PCA scores to good and bad batches, which could then be used to predict media performance.
SUB Technology
Thomas Ryll, Ph.D., senior director, cell culture development, Biogen Idec, discussed Biogen Idec’s experience with single-use bioreactors (SUB) at the meeting.
SUB technology has advanced significantly in the last decade with many different formats now available. “We settled on a more classical design of a stirred tank based disposable reactor system,” he explained.
“A question we addressed early on was whether or not a disposable reactor system would offer sufficient mixing and mass transfer to support an intensive fed-batch culture. What I mean with intensive fed-batch culture is a culture that features high cell mass (maybe 20–40 million cells/mL) and thus requires a lot of oxygen delivery.”
Mass transfer and mixing studies conducted were positive, demonstrated equivalent blending times compared to control reactor systems, and could deliver sufficient mass transfer. “We showed that 250 L and 1,000 L SUB reactors were able to grow high cell mass and could reach high titers in the 8 g/L range,” said Dr. Ryll.
“Not every system may be able to deliver such mass transfer, and the user may want to conduct mass transfer studies and may need to tailor the system selected to the purpose in mind,” he cautioned.
A few years ago Biogen Idec also began exploring technologies for online data acquisition to better monitor and understand culture behavior. These included automated sampling and inline metabolite analysis, automated oxygen uptake rate determination, culture capacitance, and NIR and Raman spectroscopy.
Raman spectroscopy was judged to have the most potential and maturity to be developed into a real-time monitoring tool in a manufacturing environment. “We’ve been able to build models that can predict metabolite levels and cell mass online, and the idea is that this adds to our tool box in terms of culture understanding and perhaps will enable us to reduce and hopefully eliminate needs for culture sampling in the future,” said Dr. Ryll.

Biogen Idec utilizes single-use bioreactor equipment in many of its cell culture optimization activities. [Thermo Fisher Scientific]
CD Supplements Can Lower Costs
“Much of the focus over the past 10 years centered on increasing the duration and cell density achieved in cell culture processes. The target is to increase the integral viable cell number (IVC)—or, as it’s sometimes referred to, the cumulative cell hours (CCH)—in order to increase and maintain the productive cell population,” said Samuel Denby, Ph.D., applications and scientific manager, BD Biosciences.
Advanced CD-defined media can boost productivity, said Dr. Denby. “In developing next-generation CD supplements, BD has identified cellular ‘activators’ that can increase specific productivity. The result of this process is to make each cell more productive, rather than just growing an ever increasing number of cells.”
“In comparing the cost of CD supplements versus peptones, we considered the process as a whole,” said Dr. Denby, “and looked at a number of qualitative benefits, including a lowered batch failure rate (through consistency of raw materials and upstream process output), more efficient facility usage (since upstream titers should be more predictable), and increased re-use of purification equipment before fouling. When looked at through a lens of total cost in use and time to market, CD supplements can provide significant benefits.” BD’s analysis revealed manufacturing cost savings up to 21%.
Regeneron’s Speed-to-Clinic Process
“Our expression technologies are quite unlike any company out there,” said Kevin Bailey, Ph.D., vp preclinical manufacturing and process development, Regeneron Pharmaceuticals. “Our speed-to-clinic cell-line technology uses a single gene copy, heavy chain and light chain, in a targeted integration locus.”
Regeneron is able to quickly generate CHO stables “such that when we choose our final antibody we actually have our cell line for early-stage clinical manufacturing. We can get in excess of 2 g/L with this technology, which for us is more than clinically enabling. It allows us to move our programs into tox production and clinical production very quickly. Our INDs, like everyone’s, are gated by production of materials for toxicology studies,” Dr. Bailey said.
While early-stage clinical work proceeds, Regeneron works on a second-generation cell line using a more conventional approach. “We don’t use a targeted locus integration for high copy cell lines but do use a repressible (inducible) cell-line approach. This allows us to isolate lines and bank them in the absence of selection pressure. We have a small molecule repressor present during that stage. For manufacturing we remove the repressor and get full gene activation and very high titers.”
Adopting a quality by design approach has been useful according to Dr. Bailey. “It guides our efforts to ensure we understand all critical quality attributes and meet necessary comparability requirements for our late-stage cell line and process.”