May 1, 2009 (Vol. 29, No. 9)
Human iPS Cell-Derived Cardiomyocytes (and other Cell Types) Streamline Quest for Novel Drug Candidates
Novel pharmaceutical discovery and development is an expensive, difficult, and inefficient process, primarily due to the lack of models that accurately present the appropriate condition or that reflect the appropriate response. Ascertaining potential efficacy and off-target toxicity is a large portion of the development process, often accomplished through the use of nonhuman animal models.
Translating the results of such experiments to the human condition is a challenging and imperfect science. Human pluripotent stem cell technology offers the potential to reduce the burden of these factors and a primary goal of Cellular Dynamics International (CDI) is to harness this technology in order to decrease both the time and cost of bringing newer, more effective, and safer therapeutics to market.
Stem cells are pluripotent cells that give rise to all the cells of the body and thus provide a potential in vitro source of any cell type. Human embryonic stem (hES) cells have already been used to generate fully differentiated cells that can be used in both drug discovery and toxicity testing, and automated methods are being applied to their culture and differentiation, thus enabling a potentially unlimited supply of any cell type and reducing the need for animals as test material.
Furthermore, the advent of induced pluripotent stem (iPS) cell technology, whereby fully differentiated cells are reprogrammed to a stem cell-like state, makes it possible to generate any cell type from any genetic background. This article will provide brief examples of current practices and future directions for the use of stem cells in drug discovery.
Cardiovascular System
While the cardiovascular system is a promising therapeutic target, it also presents significant safety hurdles. The primary advantages of human pluripotent stem cell (hPSC, which includes hES and iPS cells)-derived cardiomyocytes for use in drug discovery are: they are a human-based model; they display normal cardiac characteristics; and they survive under cell culture conditions for extended periods.
These factors are expected to enable more physiologically relevant efficacy studies as well as toxicity assessments for both electrical and traditional cytotoxic endpoints such as cell viability, ATP production, and mitochondrial toxicity.
Action potentials are the rhythmic electrical oscillations of cardiomyocyte membrane potential that underlie the heartbeat and basic cardiac function. The action potential waveform results from ions crossing the plasma membrane through a variety of ion channels. Drug interactions with cardiac ion channels resulting in action potential prolongation are of particular concern as they can lead to ventricular arrhythmias and sudden death.
The most common cause of drug-induced cardiac action potential prolongation is drug block of the hERG channel. Because the cardiac action potential waveform and ion-channel expression patterns are species specific, different toxicity assessments can be drawn from applying the same compound to cardiomyocytes from nonhuman species.
CDI’s hPSC-derived cardiomyocytes allow for an early indicator of drug-induced ion-channel dysfunction in a human model system (Figure 1). Single hPSC-derived cardiomyocytes were subjected to perforated-patch voltage clamp analysis. Action potentials were recorded from single cells under basal conditions and in the presence of terfenadine, a nonsedating antihistamine that was pulled from the market in 1998 for its potent hERG block and potential to cause sudden cardiac death.
Figure 1a shows representative patch clamp recordings of cardiac action potentials under basal conditions, during perfusion with 20 nM terfenadine, and following compound washout. Myocytes show action potential waveforms typical of ventricular hPSC-derived cardiomyocytes under basal conditions. Addition of 20 nM terfenadine causes action potential prolongation (2 min postdrug addition) that can lead to secondary premature depolarizations termed early-after depolarizations (EADs; 10 min post drug addition), which are considered a potential trigger for life-threatening cardiac arrhythmias.
Upon drug washout the action potential returns to its basal phenotype. The mean increase in action potential prolongation at 90% of repolarization (APD90) as a function of drug exposure duration is shown in Figure 1b, while the mean increase in APD90 at ten minutes of drug exposure is shown in Figure 1c.
As cardiomyocytes are aerobically poised, contractile, and metabolically active, they may present a predisposition to toxicity endpoints such as viability, apoptosis, ATP production/metabolism, and mitochondrial dysfunction. hPSC-derived cardiomyocytes are readily amenable to assessing these endpoints through commercially available test kits.
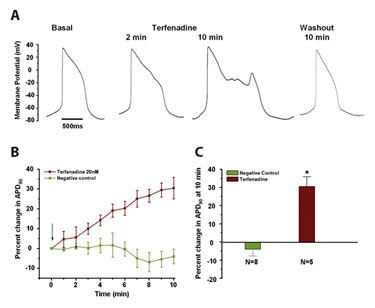
Figure 1. The effects of terfenadine on hPSC-derived cardiomyocyte action potential duration
CDI’s hPSC-derived cardiomyocytes were also used in basic cell viability and apoptosis assays (Figure 2). Cells were incubated for approximately 96 hours in the presence of increasing concentrations of one of two experimental compounds, and cell viability was assessed with a calcein-AM based live/dead kit. Both compounds significantly reduced cell viability (Figure 2a) while the negative control did not (data not shown). Additional experiments examining the underlying toxic mechanism are shown in Figure 2b.
Cells were incubated in increasing concentrations of experimental compound or positive control for approximately 24 hours, and apoptosis was assessed by measuring Caspase 3/7 activities. In this case, although both experimental compounds showed decreases in cell viability, only one compound resulted in caspase-mediated apoptosis.
Figures 1 and 2 illustrate how relatively simple experiments from an abundant supply of human cells enabled the rapid elucidation of a toxic mechanism. These examples show how hPSC-derived cardiomyocytes can provide a single, human-based model that is capable of assessing electrophysiological, biochemical, and viability cytotoxic endpoints under acute and longer-term chronic exposures.
There are currently a limited number of stem cell lines available for the in vitro generation of differentiated cell types. The value of in vitro testing can be severely limited by the lack of physiologically relevant models, arising from nonhuman models and/or material that does not appropriately reflect the target population. Induced pluripotent stem cell technology, whereby fully differentiated cells from an easily obtainable sample (skin, hair follicle, etc.) are reprogrammed to a stem cell state, overcomes these obstacles and removes nearly all the ethical implications associated with using embryonic tissue.
As illustrated in Figure 3, iPS cell technology offers the promise of generating differentiated cell types from virtually any genetic or environmental background. In addition, these cells provide the power to exponentially expand in vitro preclinical testing to fulfill the heretofore unmet needs of limited access to appropriate tissue models as well as limited diversity within those models.
Ultimately, Cellular Dynamics International’s iPS cell technology promises to streamline the drug discovery process, enabling safer and more effective pharmaceuticals to reach the clinic faster as well as enabling the clinic to more effectively use those pharmaceuticals.
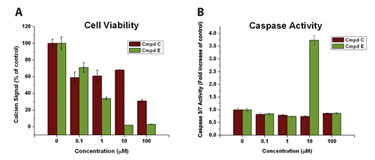
Figure 2. The effects of two experimental compounds on hPSC-derived cardiomyocyte viability and apoptosis
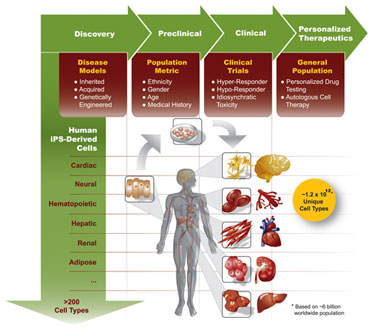
Figure 3. Induced pluripotent stem cell technology and drug discovery
Blake D. Anson, Ph.D. ([email protected]), is cardiomyocyte product manager and director of hERG screening services; Junyi Ma, M.D., Ph.D., is electrophysiology scientist, hERG screening services; and Jia-Qiang He, Ph.D., is director, ESC-cardiomyocyte validation at Cellular Dynamics International. Web: www.cellulardynamics.com.



