May 1, 2011 (Vol. 31, No. 9)
New Tool Designed to Provide Biological Relevance in Neuro-Related Drug Research
A major goal of the pharmaceutical industry has been to reduce late-stage compound attrition. Due to the immense cost associated with the drug development process, the ability to identify insufficient compounds early is absolutely critical. One area given significant focus in hopes of optimizing this process is the development of more relevant in vitro disease models.
Historically, in vitro models have played an important role in the discovery process, including early-stage disease modeling and candidate identification followed by pharmacokinetic and safety testing. These models have typically consisted of immortalized cell lines or primary cell types but have suffered from biological relevance and scalability/workflow issues. A significant push within the drug discovery field has been the development and validation of a diverse array of more relevant biological systems, including those derived from stem cell culture systems.
Much excitement arose in the drug discovery community when James Thomson and colleagues reported the derivation and expansion of pluripotent human embryonic stem cells (hESCs), as this cell type could, in theory, provide unlimited self-renewal with the retained ability to give rise to all cell types of the human body.
More recently, additional advancements by the same group and others were made whereby the addition of a specific set of four exogenous factors (i.e., OCT4, SOX2, NANOG, and LIN28) to human somatic cells resulted in the reprogramming of these cells into a state of pluripotency. These reprogrammed cells could thereafter be propagated continuously as a stem cell population referred to as induced pluripotent stem cells (iPSCs), much akin to hESCs.
Human iPSCs overcome many of the ethical issues, donor tissue limitations, and funding restrictions associated with hESCs, providing a broader audience with the means to generate pluripotent cell lines more easily and efficiently. This flexible platform opened the door for discovery models focused on specific genetic backgrounds or patient-specific disease models. Since this discovery, numerous groups have generated such human iPSC lines in an attempt to model diseases including: familial dysautonomia, spinal muscular atrophy, Parkinson disease, Huntington disease, Down syndrome, type 1 diabetes mellitus, and others.
Despite the excitement and promise associated with the use of iPSCs for drug discovery, it should be understood that the expansion of stem cells, their terminal differentiation, and ultimately, the continued culture of mature progeny is a process that poses a significant technical challenge.
In addition, applying the requisite stringent culture protocols for iPSCs and their differentiated progeny to the scale required for high-throughput drug discovery applications adds an increased level of complexity. To overcome these obstacles, Cellular Dynamics International (CDI; www.cellular?dynamics.com) has focused on harnessing the potential of iPSCs by developing robust, scalable procedures that facilitate the commercialization of highly pure, functional iPSC-derived differentiated cell types.
These novel cellular models, tested using rigorous phenotypic and functional assays, have been developed to meet the demands of pharmaceutical and academic customers alike. More importantly, the cells produced from this pipeline provide a more relevant human model system while also delivering the consistent product supply and performance required to facilitate large-scale compound screening within the pharmaceutical industry.
In 2009, CDI launched iCell® Cardiomyocytes, first-of-a-kind purified human cardiomyocytes that not only display the expected single cell cardiac phenotype but also possess the ability to form electrically connected syncytial layers with characteristic electrophysiological and biochemical responses to exogenous agents. Researchers have incorporated the use of these human iPSC-derived cardiac cells into various applications, including cardiotoxic compound screening, identification and investigation of arrhythmogenic compounds, and cardiac hypertrophy studies among others.
As a result of this success, CDI has extended its human iPSC-derived cell product development to other relevant cell types for drug discovery, including hematopoietic cells, endothelial cells, hepatocytes, and neurons.
iCell Neurons
One of the newest CDI additions, iCell Neurons, represents a population of cryopreserved human iPSC-derived neurons that, upon reanimation and culture, quickly display a typical neuronal morphology as shown through the presence of dense axonal and dendritic processes (Figure 1A). These human neurons, once plated onto tissue culture plates pre-coated with standard neuron substrates (i.e., a Poly-L-ornithine (PLO)/Laminin double coating), provide an adherent single-cell morphology.
Post-thaw, iCell Neurons develop branched networks within 24 hours and remain viable and adherent for an extended period in culture (≥14 days). The stable morphology of iCell Neurons is an important attribute for drug discovery, as it facilitates the use of these cells in various assay platforms.
Phenotypically, iCell Neurons represent a highly pure population as assayed through immunostaining for the presence of class III beta-tubulin (TuJ1) and the absence of the type VI intermediate filament nestin, a marker of neural stem/progenitor cells. As quantified through flow cytometry (Figure 1B), iCell Neurons display >90% positive staining for TuJ1 with very low levels of nestin immunopositivity.
This population of neurons is predominantly composed of a mix of GABAergic and Glutamatergic subtypes as tested through staining for the mature synaptic markers vGAT (vesicular GABA transporter) and vGLUT2 (vesicular glutamate transporter 2) (data not shown). In addition, double immunostaining of iCell Neurons for the microtubule-associated protein 2 (MAP2) and the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) reveals ~50% of the population displaying a GABAergic phenotype (Figure 1C).
To serve as a viable neuroscience discovery model, it is imperative that iCell Neurons provide a robust, consistent, and highly pure cell product amenable to common discovery applications. These applications may include high-content imaging, automated electrophysiology, and cell-based assays.
For example, iCell Neurons cultured for 7–14 days post-thaw on PLO/Laminin pre-coated 96-well plates exhibit an expected sensitivity to known compounds including the ATP-competitive kinase inhibitor staurosporine and the phenothiazine antipsychotic chlorpromazine as assayed using the Cell-Titer-Glo® Luminescent Cell Viability Assay (Promega), a measure of metabolically active cells (Figure 1D).
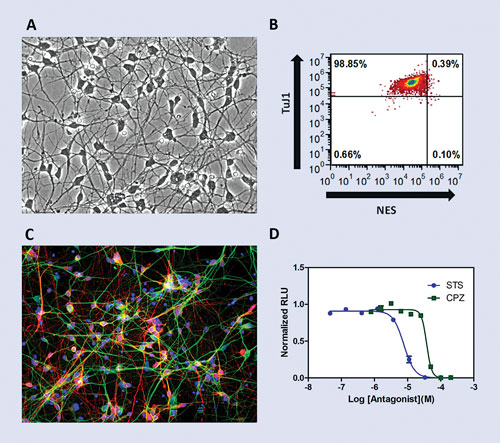
Figure 1. Phenotype of post-thaw iCell Neurons: (A) Brightfield image of iCell Neurons at day 5 post-thaw. Magnification=200X. (B) Flow cytometry for class III beta-tubulin (TuJ1) and nestin (NES) at day 1 post-thaw. (C) GABAergic neurons expressing GABA (red) and the neuronal marker MAP2 (green). Nuclei stained using Hoechst (Blue). Magnification=200X. (D) iCell Neurons display a toxicity dose-response to known compounds including staurosporine (STS) and chlorpromazine (CPZ) as measured using the CellTiter-Glo Luminescent Cell Viability Assay.
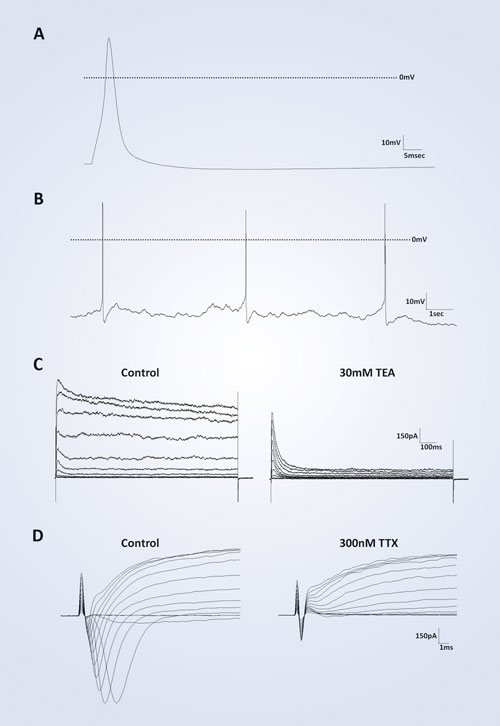
An analysis of electrophysiological characteristics reveals that iCell Neurons possess a functional phenotype. Evoked action potentials from these cells display an average resting membrane potential of -46 mV within 17 days post-thaw (n=12). All action potentials demonstrate an overshoot of the depolarization phase above 0 mV and an undershoot of the repolarization phase below baseline before correction to steady-state (Figure 2A). Also, iCell Neurons are capable of firing spontaneous action potentials with the same characteristics seen in the evoked action potentials (Figure 2B). Addition of the classic neuron ion-channel antagonists tetraethylammonium (TEA, 30 mM) and tetrodotoxin (TTX, 100 nM) effectively block outward potassium and inward sodium currents, respectively (Figure 2C, D).
In summary, CDI’s iCell Neurons represent a robust in vitro cell model, not only providing the expected phenotype associated with human neurons but also possessing functional electrophysiological attributes. These human iPSC-derived cells provide a more relevant alternative to the current cell models commonly employed in drug discovery workflows.
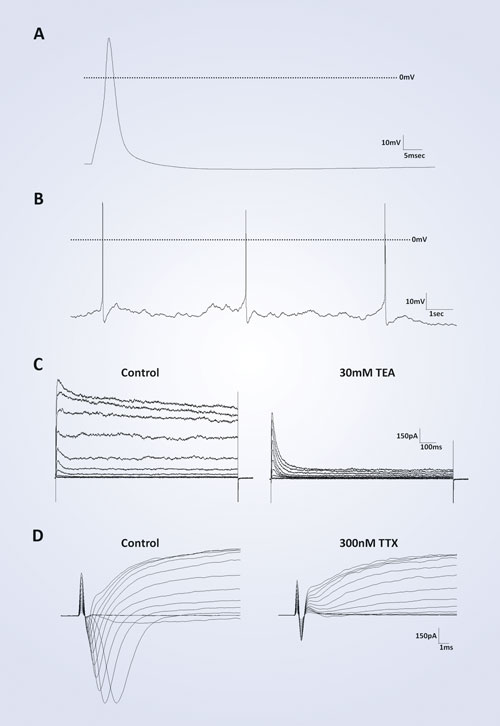
Figure 2. Single-cell neuron electrophysiology: (A) A representative evoked action potential from post-thaw day 11 neuron. (B) Representative spontaneous action potentials from a post-thaw day 14 neuron. For (A) and (B), the dashed line indicates 0 mV. (C) Potassium channel blocker tetraethylammonium (TEA) blocks outward current of post-thaw day 12 neuron from a holding potential of -80 mV. (D) Sodium channel blocker tetrodotoxin (TTX) blocks inward current of post-thaw day 13 neuron from a holding potential of -70 mV.
Lucas Chase, Ph.D. ([email protected]), and Xuezhu Feng, Ph.D., are senior scientists, David Majewski, Monica Strathman and Jeff Grinager are research specialists at Cellular Dynamics International.