March 15, 2009 (Vol. 29, No. 6)
Industry Success Requires that New Businesses Be Started to Replace Those Bought by Pharma
England’s Greater South East (an area including both Brighton and London) is the biggest health technology cluster in the world, according to David Parry, Ph.D., CEO of the South East Health Technologies Alliance. It is home to 4,700 biotech, diagnostic, medical device, and pharma companies, generating £100 billion ($142.6 billion) in revenues annually, and can also boast four of the top ten universities in the world, Dr. Parry said at a recent bioProcessUK meeting.
Other pronouncements at the meeting included that of bioProcessUK director Tony Bradshaw, Ph.D., who said that he expected the upcoming “review and refresh” of the Bioscience 2015 report to be even more supportive of bioprocessing.
Malcolm Rhodes, Ph.D., technical director at bioProcessUK, reflected on significant developments like the purchase of CAT and MedImmune by AstraZeneca. He also noted that the U.K. biological medicines pipeline is steady, and capability for Phase III has increased; however, other countries such as Germany and Denmark are also rapidly increasing their pipeline. Bottom line, the U.K. needs to start new businesses to replace those that are being bought by pharma. “We cannot afford to be complacent,” he added.
Mark Bustard, Ph.D., technical manager at bioProcessUK, highlighted the importance of knowledge transfer partnerships and the collaborative R&D program of the Technology Strategy Board (TSB, a business-led public body promoting technology and innovation for U.K. business that funds bioProcessUK). bioProcessUK assisted the TSB in assigning £10 million ($14.25 million) in funding for cell therapy projects in 2008; the next call is for highvalue manufacturing projects, which are an excellent fit for bioprocessing.
Rosemary Drake, Ph.D., CSO of The Automation Partnership (TAP), explained how the company had benefited from TSB Collaborative R&D funding to develop the Sonata—a shake-flask approach for bioprocess optimization. “It has become increasingly difficult to develop complex solutions using conventional funding approaches,” she said. “The development of a cell culture system can cost millions and take several years. VCs rarely invest in capital equipment and, although a consortium approach is valuable, it is hard to align more than three companies. For Sonata, TSB supplied about £440,000 ($674,064), matched by £1million ($1.4 million) from TAP.
“This has been important for our strategic development,” Dr. Drake added. TAP has partnered in two more TSB projects—one with Intercytex on autologous cells for regenerative medicine and another with UCL on rapid automated fabrication of tissue for corneal stem cell therapy.

The Technology Strategy Board provided funding for the development of The Automation Partnership’s Sonata, which was designed to automate the cell culture of mammalian or insect cells growing in suspension in shake flasks.
Cancer Research
Bioprocessing encompasses many different types of product. Rob Williams, Ph.D., head of development projects at Cancer Research UK, said that cancer is the biggest area for drug discovery and development with potential therapies now including vaccines, targeted small molecules, antibodies, and siRNA—and there is also a huge emphasis on biomarkers.
“Many believe that therapeutic vaccines are the holy grail in cancer,” he said. “This is a huge interest area because of relatively low cost and the specific ability to target a disseminated disease.”
Cancer Research UK spent £333 million ($474.5 million) on research in 2007–2008 and is currently involved in several cancer vaccine projects.
One is a the MFEz T-cell Phase I study that involves the adoptive transfer of autologous tumor antigen specific T cells with preconditioning chemotherapy and intravenous IL-2 in patients with advanced carcinoembryonic antigen positive tumors. In the future, Dr. Williams thinks there will be more patient stratification using biomarkers for both clinical trials and disease management and increased use of flexible trials rather than the conventional Phase I/II/III paradigm.
A key message from the TGN 1412 catastrophic incident that occured in 2006 is that studies in nonhuman primates will not always predict a safe starting dose in man when a novel agent is concerned. Therefore, Cancer Research UK has started various clinical trials on novel agents using different data such as surrogate antibodies, knock in/out animal models, and human cells as a preclinical package.
Duncan Casson, Ph.D., COO of PanGenetics, said that TGN 1412 had been the 9/11 of antibody development; security and regulation have become even more stringent since.
PanGenetics was launched in 2005. PG102, its lead compound, is an anti-CD 40 antibody with a unique mode of action for autoimmune, rheumatoid arthritis, and Crohn’s disease, Dr. Casson noted. The company is also working on PG110 an anti-NGF antibody for chronic pain. “Most antibody companies set up a platform and take everything forward,” he said. “We turned this on its head and went for the low risk ones, PG 102 and PG110.”
Time to clinical proof-of-concept is increasing because of an increase in the regulatory burden and a scarcity of biologics-naive patients and of reliable and competent contractors, Dr. Casson added.
Antibody Development
Paul Varley, Ph.D., vp development at MedImmune, discussed the role of bioprocessing in antibody development. “Bioprocessing holds the key if antibodies are to reach their full potential as drugs,” he said. In the last 10 years, there have been tremendous advances in yields and ease of purification.
Higher titers have improved plant capacity and lowered costs—but gains are limited by downstream issues and fixed overheads. The consistency and complexity of antibodies remain a significant challenge, but advances in analytical science and process development are providing a better understanding of, for instance, process parameters and product attributes. Moreover, troubleshooting is now faster and more robust.
Disposables are also helpful because they increase flexibility, allowing MedImmune to go to fast early production of lead candidates (they have around 100 antibodies in their portfolio). “We want to have process knowledge from day one,” Dr. Varley said. In regulatory development, he sees an increasing interest in quality by design and MedImmune is now working on this with the FDA. He also notes increasing acceptance of platform processes by regulatory authorities, which has the benefit of getting more products with fewer resources.
TGN 1412 had a dramatic influence on the fortunes of another antibody company. Andy Porter, Ph.D., professor of biotechnology at the University of Aberdeen, founded Haptogen as a spin-out. The firm had to abandon IPO plans after the TGN 1412 fiasco. Further development, however, including Sharp®, a shark-antibody platform licensed in from the University of Maryland, and a tight focus on infection and inflammation, enabled Haptogen to survive. It was acquired by Wyeth in 2007.
Dr. Porter is also involved in ImmunoSolv, a spin-out from the University of Edinburgh Medical School. Dead-Cert™, which measures apoptosis and can reportedly improve cell populations through dead cell removal, is the firm’s antibody and nanoparticle technology.
Apitope, which stands for antigen processing independent epiTOPE, also presented at the bioProcessUK meeting. It is developing peptide therapeutics for autoimmune disease; the firm currently has a multiple sclerosis candidate in the clinic.
Cell Medica, funded by Imperial Innovations and the Wellcome Trust, has discovered a way of crossing the HLA barrier in cell therapies for infectious disease. Its lead therapy targets prevent of infection like cytomegalovirus (CMV) in immunosuppressed patients after allogeneic bone marrow transplant by selecting CMV-specific memory T cells from a donor.
Fusion Antibodies, a spin-out from Queen’s University Belfast, is focused on discovery, development, and commercialization of antibody-based therapeutics for cancer and angiogenesis. Its Fusion Expression Technology ™ can deliver the most challenging proteins, according to Paul Kerr, Ph.D., director of business development.
MedCell is focused on using stem cells for musculoskeletal regenerative therapies. Its key interest is in 3-D bioprocessing involving scaffolds without growth restraints. “This is a more natural environment for cells to grow in,” said Wesley Randle, Ph.D., program director. NovaPod is a research-grade disposable bioreactor that allows the culture of cells in three dimensions.
Medella™ Therapeutics, a spin-out from the University of Sheffield, is working on drugs that interact with receptor activity modifying proteins (RAMPs) with an initial focus on cancer although there is potential application for this therapeutic principle in bone and cardiovascular disease, CNS, inflammation, and obesity.
“This is a novel biological target,” said Gareth Richards, Ph.D., senior scientific officer. The company is working on adrenomedullin, the ligand for RAMP2 and RAMP3, which is expressed in 80% of tumors and whose expression increases in hypoxic conditions; it can also encourage an aggressive tumor phenotype. Medella’s RAMP3 antagonists can target the disease functions of adrenomedullin and it already has proof of principle of this with an in vitro increase in apoptosis and an in vivo shrinkage of tumor volume.
Finally, Stabilitech based at the Imperial College Incubator, London, has developed a technology for the thermal stabilization of live viral vaccines (enveloped and nonenveloped) and other biologics to allow for long-term storage over a wide temperature range.
“The idea comes from the way seeds solve the problem of stabilizing complex proteins through deposition of sugars and selective expression of certain proteins,” explained CEO Barbara Domayne-Hayman, Ph.D. The firm mimicked this with low-cost, nontoxic, and water-soluble chemical excipients, combined with freeze-drying, to produce a technology that is easy to integrate into a cGMP manufacturing procedure, she said.
The technology removes the need for a cold chain, reduces costs, allows stockpiling of vaccines, and may allow products to be brought onto the market that would otherwise be too unstable, according to Dr. Domayne-Hayman. It has been applied to measles, adenovirus, and some other live vaccines, as well as sub-unit vaccines, growth factors such as G-CSF, and also other proteins, peptides, enzymes, and antibodies.
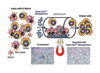
Dead-Cert nanoparticles are specially prepared to allow direct removal of dying and dead cells from cell suspensions and cultures with minimum fuss and maximum efficiency, according to ImmunoSolv.
Formulation
Formulation is, of course, a key element in bioprocessing. Jan Jezek, Ph.D., director of protein formulation at Arecor, discussed the challenges of stabilization in protein formulation. Arecor offers bespoke solutions for protein stability to pharma and biotech clients with Arestat™ offering heat stability during storage (Arestat-T) or during radiation exposure (Arestat-R). Formulation always has implications for the stability of a product.
Arecor looks at the physicochemical characteristics of every component of the formulation—for instance, is the excipient capable of hydrogen ions with the protein and are there other equilibria that might be set up? Dr. Jezek listed the challenges to stability including high concentration, aqueous solutions, virus-like particles, depot formulations, terminal sterilization, with ionizing radiation. The latter is convenient but also damaging to proteins.
Arestat-R has increased the stability of a number of proteins close to 100%, Dr. Jezek said. The product contains the right mix of excipients to stop irreversible reactions of high energy species during sterilization. “The take-home message is that it is possible to sterilize proteins and maintain 100 percent activity and structural integrity,” Dr. Jezek said.
Susan Aldridge, Ph.D. ([email protected]), is a freelance science and medical writer.



