October 15, 2008 (Vol. 28, No. 18)
New Washer Equipped with a Magnetic Bead Plate Carrier for Processing Multiple Samples
Biomedical research and, in particular, cancer research is progressing from focusing on small numbers of molecules or cellular events to global functional analysis, feeding these results into new approaches for the prevention, diagnosis, and treatment of cancer. Methods that allow researchers to look across a broader angle at cellular processes such as mRNA expression levels or protein interaction patterns are needed more often to study fundamental processes.
Coaffinity purification of two proteins from a complex mixture is one of the standard methods for the detection of protein-protein interactions. To circumvent the need for specific antibodies in affinity purification and subsequent detection, proteins can be expressed in fusion with a tag, i.e., an extension that has a high-affinity binding site for a generic antibody. When two differently tagged proteins are used, the first tag can be used for the specific purification of the complex and the second tag for the detection of the copurifying protein.
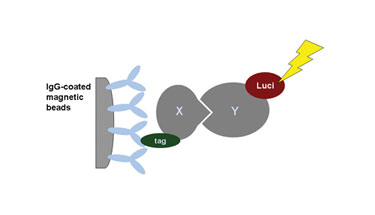
In one variation of this protocol, the tag for detection is not an antibody tag but an enzyme that can be detected via its catalytic activity such as luciferase, highly amplifying the read-out signal. This avoids the need for gels and blots for detection of the tag and allows microplate-based miniaturization and automation of the protocol (Figure 1).
In this article, we describe the use of Tecan’s HydroFlex™ washer equipped with a magnetic bead plate carrier for fast purification of a large number of samples using magnetic beads. For the detection of the luminescence signal Tecan’s Infinite® F200 multimode reader was used. In the protocol in our lab at the German Cancer Research Center (DKFZ), the affinity tag is Staphylococcus aureus protein A, which can be purified on immunoglobulins as the affinity matrix.
The use of magnetic beads for immobilization of immunoglobulins is advantageous for miniaturization and automation of the assay.
Figure 2 depicts the workflow of the experiment. After transient expression of the protein pair in tissue culture cells, a lysate is prepared and allowed to bind to immunoglobulin-coated magnetic beads. Nonbinding proteins are removed from the beads in a washing step. Luciferase activity is determined in the starting material as well as in the purified beads. The fraction of bound luciferase activity in the positive versus the negative control is taken as a readout for binding.
An important step in such an automated protocol is the washing procedure used for purification of the protein complexes. The options available in the HydroFlex protocol allow fine-tuning of the washing procedure. The relative speed of the washing steps ensures a minimal loss of protein complexes during the wash, while maintaining a high enrichment factor, as can be measured via the activity of the luciferase tag.
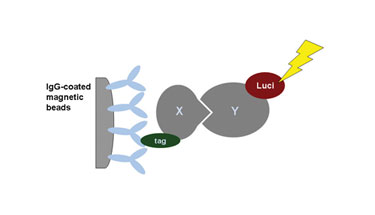
Figure 1. Principle of luciferase-based coprecipitation essay.
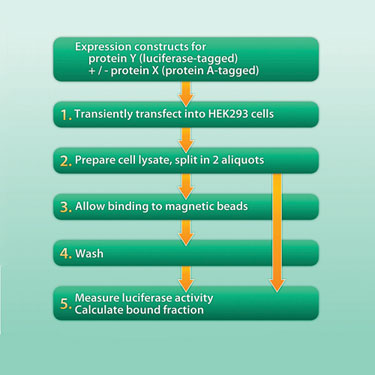
Figure 2. Workflow of a typical experiement.
Material and Methods
Instruments
• HydroFlex washer equipped with 16 channel washhead, four inlet-channels for wash buffers, and the Option smart-MBS carrier for washing of magnetic beads (Tecan)
• Infinite F200 multimode microplate reader (Tecan)
Microplates/Beads
• LumiNunc™, 96-well microplate, white and flat bottom (Nunc, part of Thermo Fisher Scientific)
• Dynabeads® M-280 sheep antirabbit (Invitrogen Dynal)
Assay Procedure
HEK293 cells were cotransfected with vectors directing the expression of proteins fused to a protein A tag as well as a luciferase-tag (Renilla luciferase). Cell extracts of transiently transfected HEK293 cells were allowed to bind to magnetic beads coated with goat antisheep IgG (Invitrogen) for two hours before washing with ice-cold phosphate buffered saline (PBS) using the HydroFlex. Washed beads were resuspended in 60 µL PBS 1 mM DTT. Luciferase activity associated with the washed beads was determined using an Infinite F200 multimode reader (attenuation: none, settle time: 0 seconds, integration time: 1,000 ms).
Results
While wash program 1 yielded an excellent enrichment as well as low background signal, there was a loss of bead material from the wells that was apparent by visual inspection. To avoid loss of beads, the following changes were introduced leading to wash program 2:
• After addition of the wash buffer, a soak time of one minute was added to allow the beads to reattach to the magnets.
• The aspiration rate was lowered to one.
• The z-position was elevated such that the liquid was not removed completely, leaving about 20 µL in each well.
To test how many cycles were needed for near complete removal of nonassociated luciferase activity, we ran the identical samples with 1, 2, 3, 4, and 5 washing cycles (Figure 3). Without the need to resuspend the beads from the well bottom during dispensing of the wash buffer, three wash cycles were sufficient to achieve an over 300-fold enrichment of bound material.
Typical binding stoichimetries of well-characterized protein-protein interactions in these experiments ranged from 0.05 to 3% of Renilla-tagged protein recovered in a complex with the protein A-tagged protein. The sensitive bioluminescence measurement allows the detection of weak interacting proteins and instable protein complexes.
The flexible programming of the washing steps possible with the HydroFlex allowed a straightforward test of optimal washing conditions. Washing efficiency is close to the theoretical optimal, despite the fact that most of the beads remain attached to the well bottom during the washes.
The low number of washing cycles is crucial for the purification of unstable protein complexes from cells. Purification can be done with the HydroFlex washer (Figure 4) in combination with the Infinite F200 multimode reader in a short time. In this way, it is possible to process up to ten plates (800 protein interaction assays) in less than an hour.
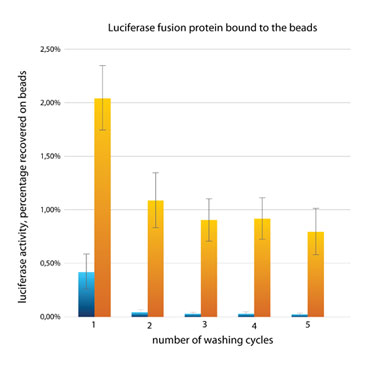
Figure 3. Testing the number of washing cycles.

Figure 4. The HydroFlex washer is equipped with a magnetic bead plate carrier for fast purification of a large number of samples using magnetic beads.
Manfred Koegl, Ph.D. ([email protected]), is head of the
protein interaction and assay development unit at Translational Research, and Genomics and Proteomics Core Facilities, German Cancer Research Center (DKFZ), Heidelberg, Germany.
Web: www.dkfz.de.